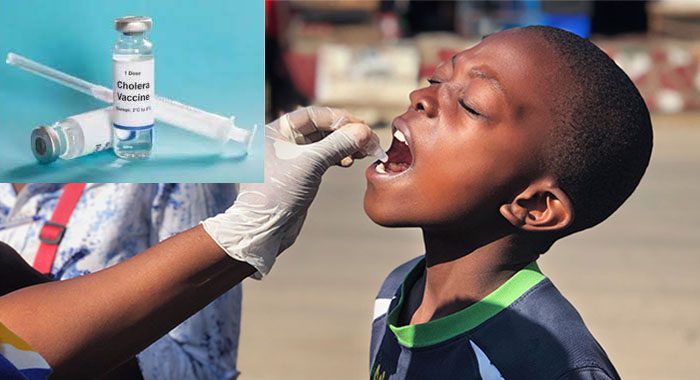
A recent oral cholera vaccine called Euvichol-S has been approved by the World Health Organisation.
The WHO made this announcement on Thursday via a press release on their website.
The approval was granted on April 12, 2024.
The purpose of WHO vaccine prequalification is to guarantee the safety and effectiveness of vaccines used in immunisation programmes. In assessing vaccines for prequalification, WHO uses international standards to thoroughly evaluate and establish their safety and effectiveness.
WHO also ensures that prequalified vaccines remain safe and effective through regular re-evaluation, site inspection, targeted testing, and investigation of any product complaints or adverse events following immunisation.
The role of national regulatory agencies and national control laboratories is crucial in WHO vaccine prequalification as they are responsible for regulatory oversight, testing, and release of WHO-prequalified vaccines.
The UN agency mentioned that the inactivated oral vaccine Euvichol-S has a comparable effectiveness to existing vaccines but with a simplified formulation, allowing for the rapid increase in production capacity.
Euvichol-S was developed through a collaboration between EuBiologics, the International Vaccine Institute, and the Bill and Melinda Gates Foundation.
The Director of the WHO Department for Regulation and Prequalification, Dr Rogerio Gaspar, stated, “The new vaccine is the third product of the same family of vaccines we have for cholera in our WHO prequalification list. The new prequalification is expected to enable a rapid increase in production and supply, which is urgently needed by many communities battling with cholera outbreaks.”
In Nigeria, cholera is a persistent and seasonal disease, mostly occurring during the rainy season and particularly in areas with inadequate sanitation.
In 2022, WHO reported 473,000 cases of cholera – double the number from 2021. An additional 700,000 cases were estimated for 2023. Currently, 23 countries are experiencing cholera outbreaks, with the most severe impacts observed in Comoros, the Democratic Republic of the Congo, Ethiopia, Mozambique, Somalia, Zambia, and Zimbabwe.
The WHO prequalification list already includes Euvichol and Euvichol-Plus, inactivated oral cholera vaccines produced by EuBiologicals Co., Ltd, Republic of South Korea, which also manufactures the new vaccine Euvichol-S.
The global health body highlighted that vaccines offer the quickest intervention to prevent, limit, and control cholera outbreaks, but supplies have been at their lowest point amidst countries facing critical deficiencies in other areas of cholera prevention and management, such as safe water, hygiene and sanitation.
Meanwhile, Gavi, the Vaccine Alliance, and the United Nations Children’s Fund welcomed the news of the new vaccine.
A joint statement by the organisations stated that the prequalification of the new product will help EuBiologics, the manufacturer, produce larger volumes of the vaccine more quickly and at a lower cost – a crucial step in expanding supply during the ongoing acute global increase in cholera outbreaks.
Today’s approval will help boost the total supply of OCV available in 2024, with around 50 million doses now estimated to be accessible to the global stockpile this year, compared to 38 million in 2023.
Euvichol-S is a significant product innovation: a simplified version of Euvichol-Plus that reduces the number of vaccine components – this creates a vaccine that studies have shown remains equally effective against key cholera serogroups while reducing production cost and complexity, allowing for faster production of larger volumes.
Gavi, UNICEF, and partners recently announced the largest-ever worldwide deployment of cholera diagnostic tests to support surveillance and response.
WHO stated that over 1.2 million diagnostic tests will be distributed to 14 high-risk countries in the next few months.
“Prequalification of Euvichol-S is vital for vulnerable communities worldwide,” explained the Managing Director of Vaccine Markets & Health Security at Gavi, Dr Derrick Sim. “Each vaccine dose delivered through Gavi programs today represents years of planning and investment to match supply with countries’ needs.
“The approval of this new product is extremely timely given the sharp increase in cholera outbreaks we are witnessing worldwide. We commend EuBiologics for their role in ensuring countries have access to cholera vaccine as part of their response toolkit.”
The statement added that EuBiologics is currently the sole supplier of OCV to the global stockpile, but other manufacturers are expected to have products available in the coming years.
“Gavi, the Vaccine Alliance works to shape the OCV market, and funds the global stockpile of OCV doses, along with transport and vaccination activities in lower-income countries.
“UNICEF leads on procurement and delivery of doses to countries. Use of the stockpile for emergency response is overseen by the International Coordinating Group for Vaccine Provision, led by WHO,” it added.
The Director of UNICEF Supply Division, Leila Pakkala said, “Despite cholera being preventable and easily treatable, children continue to suffer from this potentially fatal disease. UNICEF has already secured access to all the available doses of the just-approved vaccine and will deliver these to the countries at the highest possible speed.
“The approval means that UNICEF can increase the procurement and delivery of cholera vaccines by more than 25 per cent, pushing back harder on deadly cholera outbreaks.”