It’s time to welcome a new type of cell to the group of living things that can gather nitrogen from the atmosphere.
Until now, only bacteria and archaea were believed to pull nitrogen from the air and convert it into a useful form. But scientists have found a specific ammonia producer within a single-celled ocean plant that adds organisms with membrane-bound structures called organelles to the list, researchers report in the April 12 Science.
The scientists say that this producer was once a bacterium that started living inside the plant about 100 million years ago and has since evolved into a nitrogen-gathering machine for its host. Once a symbiont, it’s now one of the cell’s many organelles.
Nitrogen fixation, where atmospheric nitrogen gas is transformed into ammonia, is a crucial process for life (SN: 4/28/17). Organisms need access to nitrogen-containing compounds to create essential biochemicals. The bacteria and archaea that have this ability often carry out gas refinement in soil or in aquatic environments like the ocean.
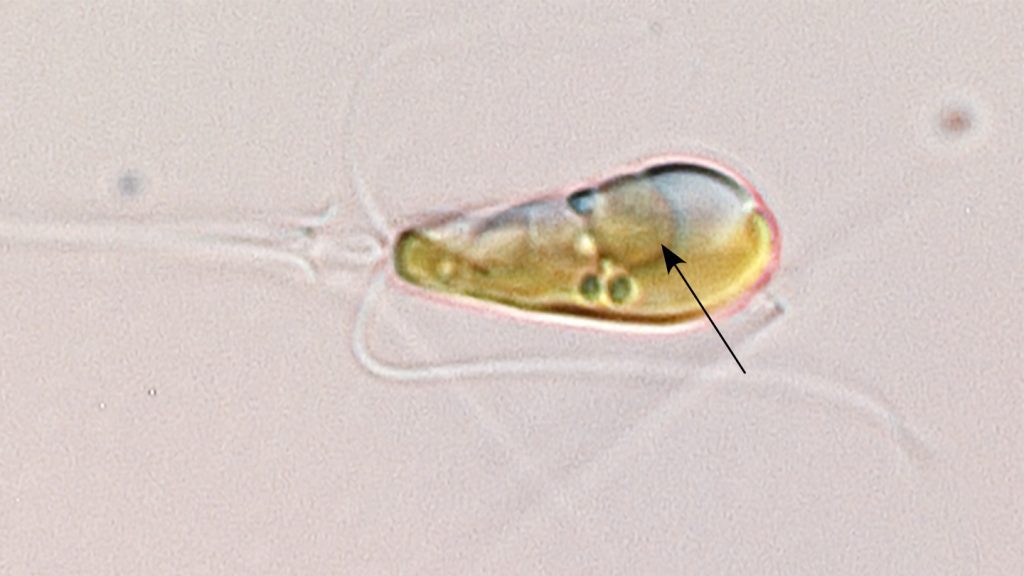
One such bacteria, called UCYN-A, is widely distributed in the world’s oceans and plays a key role in oceanic nitrogen fixation, says marine ecologist Jonathan Zehr of the University of California, Santa Cruz. These bacteria also serve as symbionts within the single-celled algae Braarudosphaera bigelowii and its relatives.
However, it can be difficult to distinguish between symbiont and organelle. Zehr and colleagues aimed to gain a better understanding of where UCYN-A falls on that spectrum.
Using X-ray imaging, the team first discovered that when the plant cells divide, all its organelles line up and take turns dividing in a defined sequence. “This symbiont participates in that sequence,” says marine biologist Tyler Coale, also of UC Santa Cruz. “It’s somehow getting the cue to divide right on time with the other organelles.”
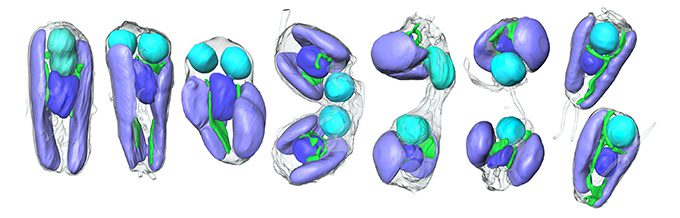
Next, the researchers analyzed the complete sets of genetic instructions and proteins — the genomes and proteomes — made by the algae and the UCYN-A symbionts. “About half of the proteins that are physically present inside this symbiont come from the host genome,” Coale says. These additional proteins appear to fill gaps in the bacterium’s crucial metabolic pathways, indicating it relies on the plant cell’s proteins to function.
In line with this, many of the bacterial proteins contain special amino acid chains. Molecular biologist John Archibald, who was not involved in the study, describes them as “postage stamps” for transporting proteins within the cell. A similar system exists for directing proteins that are encoded by the host cell’s genome into mitochondria and chloroplasts — organelles believed to have developed from helpful microbes (SN: 11/5/18).
“The information clearly indicates that the two cells have been evolving together for quite some time,” says Archibald, of Dalhousie University in Halifax, Nova Scotia.
The researchers argue that all these characteristics indicate UCYN-A isn’t just a symbiotic organism but has transformed into an organelle: the nitroplast.
“Protein import is really the convincing evidence,” says Oliver Caspari, a molecular biologist at the University of Bonn in Germany who was not involved with the study. That import implies a level of interdependence that marks the bacterium as an organelle, he says.
The nitroplast is one of only four known cases of symbiotic microbes becoming key parts in a host’s cellular machinery. In particular, chloroplasts and mitochondria evolved from microbial symbionts as much as 2 billion years ago. Previous research on the evolutionary history of UCYN-A showed its connection with algae is much more recent — around 100 million years old.
That means nitroplasts may provide a snapshot of how mitochondria and chloroplasts evolved into organelles (SN: 11/5/18). Researchers have long thought that this process involved symbiont genomes migrating into the nuclear genome of the host, but there doesn’t seem to be evidence of this in the nitroplast, Coale says. Instead, the host’s genome may support the symbiont to the point that the symbiont’s own genome withers away.
“If genes are targeted and their proteins are imported into these organelles, then their genomes are free to lose those genes,” Coale says. “Maybe this is the mechanism by which symbionts are domesticated.”