Jerry Coyne is an evolutionary biologist at the University of Chicago. His research on population and evolutionary genetics has been widely published in professional and trade journals and his 2009 book, Why Evolution Is True, established him as a leading force in the study of evolution. Jerry is also an internationally famous defender of evolution against proponents of creationism and intelligent design. He is a highly respected scientist.
This, however, is a more personal story about Coyne. It goes back to 1973, when he was a mere 24-year-old graduate student at Harvard. As he moved through the program, Coyne was becoming well versed in the intellectual tools of his trade—genetics, evolutionary logic, research methods, and the like. But when it came to real-life contact with nature, his experience was pretty much “limited to unexciting fruit flies crawling feebly around food-filled glass tubes.”1 He was even more frustrated working at Harvard’s Museum of Comparative Zoology. This was the same museum that was founded by the great Swiss naturalist Louis Agassiz, under the guiding philosophy to “study nature, not books.” But, aside from fruit flies in a sterile lab, the only nature Coyne was seeing were stuffed mammals in a display case on his way to the Pepsi machine. When given the opportunity to take a summer field course in tropical ecology in Costa Rica, Coyne didn’t hesitate. He never imagined how close to nature he would get.

Toward the end of his stay in Costa Rica, Coyne was walking through the forest when he heard a mosquito getting closer and closer, and finally, it bit him on the head. “Not too far from the crown and I scratched it,” he recalled.2 But, unlike a usual mosquito bite, this one didn’t want to go away. When, after a few days, the bump had grown to the size of a pea, Coyne consulted with a fellow student who was an entomologist. His friend got up on a bunk bed. “She looked at my head and pulled the hairs back, and she said, ‘Oh my God, there is something moving in there,’ ” Coyne said. She spotted what appeared to be a tiny hose protruding from the mosquito bite. Then she realized the hose was wiggling. It was a breathing tube, like a little straw. That meant there was something live on the other side of the tube. The two biologists knew right away it had to be a maggot.
The maggot turned out to be a botfly, a hairy insect that lives in tropical regions in Central and South America. It has a biologically ingenious and, most humans would think, rather disgusting strategy for ensuring the survival of its young. The process works something like this: After a pregnant female lays her eggs, she flies in the air and grabs on to a mosquito. Then, in midflight, she glues her eggs to the mosquito’s wings. The mother leaves. The mosquito, who probably has no idea anything has happened, continues doing what it always does, which is to fly around until it finds a warm mammal and sucks its blood. When the mosquito finds its prey, the mammal’s heat triggers the eggs to hatch. One of the newly hatched larva—a tiny maggot—burrows its way inside the mammal through the mosquito bite, sets up a little home, and sticks its breathing tube out the opening. Botflies feed on the mammal’s tissue until, after about six weeks, they’ve grown big enough to survive on their own, and then they exit through the hole in their host’s skin. Coyne happened to be the host this time around.
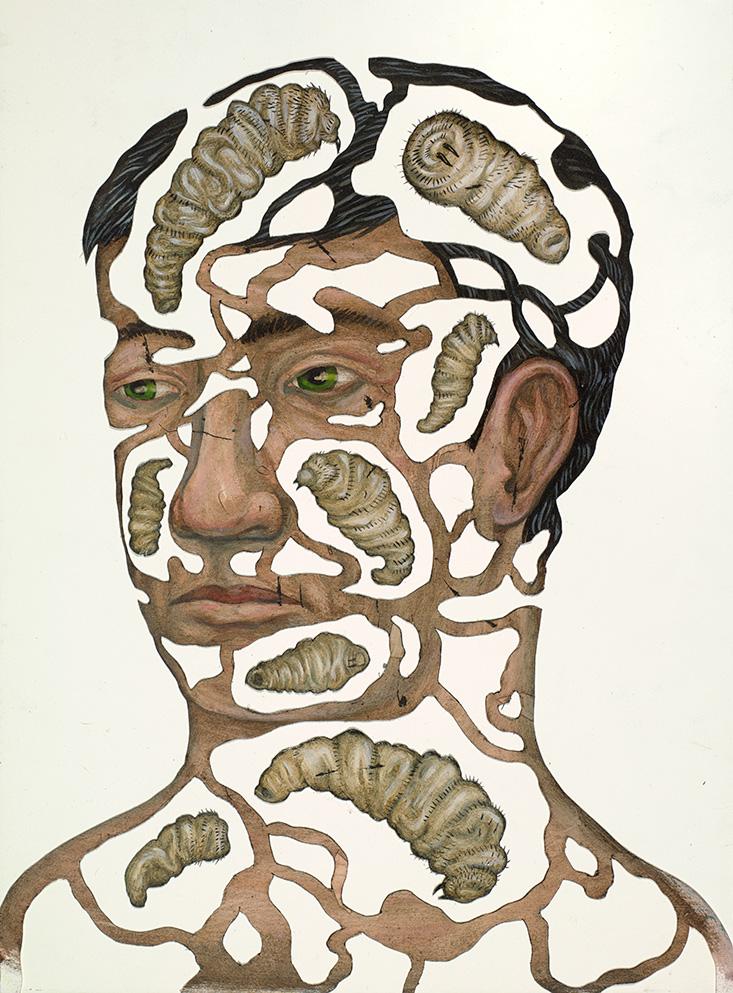
The maggot raises difficult questions about the division between self and non-self.
He soon learned removing the botfly wouldn’t be easy. The best solution would have been to cut it out with a sterile scalpel. But finding a good surgeon was a problem in a remote tropical forest. “There was a woman in the course who had botflies in her butt,” Coyne recalled to me. She found someone to surgically remove them, but it wasn’t a pretty scene. “This guy took her in a back room and started cutting them out with a Swiss army knife. I remember we could all hear her screaming when this was happening. I found myself thinking, ‘Do I really want to go through this?’ ”3
He was tempted to try yanking the maggot out by pulling on its protruding spiracle. But Coyne knew that was probably the worst thing he could do. “Like all marvels of evolution, the botfly maggot has devices to keep you from pulling it out because it makes its living in your body,” he explained. “So it has a pair of hooks on the anal end, the other end, that are dug into your flesh so if you try to pull the thing out it just digs in and you’ll break it in two. That’s the thing you want to avoid because it can cause a serious infection.”4
The most common treatment where Coyne was living was known as the “meat cure.” He was told to strap a slab of meat—a steak, maybe—to his head. This cuts off the maggot’s air supply, and the maggot, thinking the steak is part of Coyne’s flesh, burrows into it searching for air. Once the maggot gets far enough, he would just have to pull off the steak with the worm in it. It made sense, but Coyne respectfully declined. “The idea of toiling in the tropical heat every day with a T-bone strapped to my head was not something that I wanted to do.”
Meanwhile, the symptoms were getting worse. “It’s a terrible itch and from time to time it would like move or twitch and you would feel this sort of sharp pain in your skull or you could feel it grinding up against it,” Coyne recalled. “And when I went swimming or took a shower, it would sort of freak out because its airhole would be cut off, then it would really go nuts. You know, make a lot of pain. So I tried to avoid getting my head under water.”
The lump was also getting noticeably bigger, and Coyne was all too aware why. “It was eating my muscles and tissues and scalp,” he said. “It’s turning human flesh into fly flesh.” Like any normal person, Coyne was initially disgusted. “I freaked out completely,” he said. But then the scientist in him took over. This was biology in its elegance. “You know, when you really think about it, it’s amazing how an animal can take human flesh and turn it, using its own genes, into a fly.”5 How astonishing, he thought, that “something was transforming my molecules into their own. The idea that a fly could convert you into a fly. That really amazed me.”6 And the fact that this fly, by eating him, was in a very literal sense becoming him? “That’s the part that made me like it,” he says.7
Coyne returned to Boston a few weeks later and went straight to the Harvard health clinic. “Nobody had ever seen anything like this at Harvard,” Coyne recalled. Within minutes he was surrounded by about 20 doctors. “I had to explain it to them. They were all poking and prodding but none of them seemed to know what to do with this. So I figured it wasn’t worth it to put myself in the hands of people who’d never treated anything like this before and were more likely to screw it up. The botfly wasn’t that painful and I knew it was going to come out on its own after a while,” Coyne told me.8 He decided to just try to enjoy and marvel at what was happening inside him as much as he could.
“This behavior might seem weird to a lay person,” he said, but “I make my living on flies. I work with fruit flies. I’m a geneticist, and here is a fly making its living on me.” Coyne was intrigued to find himself inside a food chain instead of on his usual perch as a consumer at its end. The botfly was fattening up on Coyne, and Coyne was becoming increasingly fond of the botfly. “I was getting more and more curious when it would come out. I didn’t want to kill it.”9
The botfly kept growing. Within a couple of weeks it had become the size of an egg, then a quail egg. Coyne started wearing a baseball cap. One night he was at a Red Sox game at Fenway Park with his friend Sarah Rogerson. “Every once in a while I would rub my head, throughout the whole gestation of this thing, just to check on it. During the game, when I rubbed my head I felt something coming out of the lump. “Jerry kept saying, ‘Oh my gosh. Oh my gosh. It’s coming out. I can feel it,’ ” said Sarah. “A foul ball came up where were sitting and it hit one of those wooden seats in Fenway and we narrowly escaped getting hit because we really weren’t paying much attention to the game at all.”10
What if the visitors didn’t simply destroy you but fundamentally changed you?
It didn’t come out right away. Sarah and Coyne went back to his apartment. He kept checking to feel the lump. Sometime later in the evening, he reached up and said, “It’s gone. It’s out.” He told Sarah they had to find it. “I turned on the light and there it was on the pillow and it was horrifying,” Coyne said. It was a fat, white worm, about an inch and a half long. It was bulbous on one end and tapered to a little tail on the other. And it had little black teeth. Forever the evolutionary biologist, Coyne was struck by the painlessness of the exit. “You know, it’s painful when it’s in there but when it comes out it does so very painlessly.” This, he realized, was another evolutionary invention. “If the worm did it painfully, then the horse or the monkey or whoever it is infecting would just slap it and kill it.”
But mostly he wanted to save the fly. He looked at his baby on the pillow and decided to try to rear it into an adult fly. “I’d prepared a jar of sterile sand and I took the worm and dropped it in the sand and put on a top with an airhole,” Coyne said. “But unfortunately it died.”11 Looking back, he said that he was sorry he “didn’t just put it into a jar of alcohol to preserve it.”12 Coyne felt extremely sad afterward. “You know in the temperate zone in Boston the botfly is not going to make it. It just can’t live and so it was doomed from the start. I wanted to see it complete its life cycle but unfortunately it didn’t quite make it. I did the best I could with what I knew.” He felt the loss. “It added richness into my life, it really did. People still get completely horrified when I tell them the story even though to me it’s sort of a nice story.”13 And, he told me, “It was my botfly.”14

Jerry Coyne’s maggot was clearly an intruder—a hit-and-run thief who conned its way into Coyne’s body, stole what it needed, and, when Coyne was no longer useful, went off to live an independent life. It was a parasite. But the maggot also raises difficult questions about the division between self and non-self. The maggot was never invited into Coyne’s head. For those weeks it lived there, however, wasn’t the maggot in many ways literally Coyne? After all, other than the tip of its breathing tube, the maggot existed completely inside his body. Besides, the maggot was flesh-and-blood Coyne in the most literal sense: Almost the entirety of its physical bulk consisted of Coyne’s tissue. And then there was the boundary. To protect itself from infection, Coyne’s body had encapsulated the maggot in a little pocket under the surface of the skin. The pocket became the maggot’s home. The same physical boundary separating Coyne from the “lifeless” air outside himself—his epidermis—was now also separating the maggot from its own outside. So was the pocket more Jerry Coyne or maggot?
The psychologist in me loves watching the quirky ways people draw their personal boundaries. It’s interesting how emotional we get when we believe somebody or something has crossed into our personal territory. But, the fact is, psychological feelings are trivial in cases like this. It didn’t make much difference whether Coyne thought of his botfly as himself or his guest. Their relationship was a matter of life or death—certainly for the botfly and potentially for Coyne. Biologically speaking, all that mattered was that Coyne let the botfly survive.
And biologically is where the line between self and other is most critical. It is so important that we have, over the course of evolution, developed what is arguably the most complex biological system (other than perhaps our nervous system) in any living vertebrate to do the job: our immune system. The immune system is our border patrol. It consists of a roving bag of cells that patrol our body 24/7. Its job is to identify trespassers, assess their danger, and eliminate them if necessary. These watchguards are exquisitely tuned to distinguishing the fingerprints of intruders from their own body’s cells and molecules. One of their specialties is spotting parasites.
Coyne’s immune system had tracked the invader, labeled it as non-self, probably assessed that it was a temporary visitor who posed a limited danger, and decided it would be safer to contain rather than kill or remove it. Antibodies were dispatched to seal the maggot securely in a little pocket. All Coyne did was decide to let biology run its course.
The anatomy of the self sounds simple. There is my body and then there is everything foreign to my body. Not so, it turns out. When it comes to the self-other boundary, the anatomical lines can be as messy and confusing as the ones we draw in our minds. Perhaps even more so.
Consider, for example, what are known as “molecular mimics,” a type of parasite whose very specialty is confusing self and other. “These parasites are really crafty,” says parasitologist Paul Crosbie. “They get inside the host’s body and stick some of the host’s protein on the outside of their own cells.”15 It’s how they survive. Some mimics even raid the macrophages, our combat troops, the very antibody cells whose job is to kill and devour parasites. The parasites then pull off pieces of the macrophages and stick them on their own surface as camouflage. Other parasites have learned to mutate their appearance to match that of the host’s cells. As Crosbie says, these molecular mimics fool the host’s immune system into thinking, “It’s okay, that’s one of my cells.” These sneaks, these miniature secret agents, are the biological world’s masters of disguise.
Don’t go looking for the serenity of Mother Nature in the world of microbiology. This is a savage little jungle full of con men and killers. When Darwin argued that nature was a bad place to prove that God had a benevolent design, he pointed to parasites as primary evidence. “It is derogatory that the Creator of countless systems of worlds should have created each of the myriads of creeping parasites,” he wrote.16
In place of a tongue you see a slimy, multilegged creature, its beady eyes staring straight at you.
If you’re looking for a lesson in con artistry, may I recommend a group of mimics that operate as intracellular invaders? These trespassers don’t just disguise themselves as host cells. They sneak right inside the actual cells and wear the host’s membrane as their disguise. The immune system, which is a network of cells itself, only interacts with the surface of other cells. Antibodies, which are part of the immune system, don’t cross cell membranes. So once an assailant manages to get inside a cell, it’s home free. It enters a safe haven where it can grow, reproduce, and, in some cases, plan its next attack.
Species of Plasmodium, the protozoa that cause malaria, are a good example of these parasites. They enter the host’s body through a mosquito bite and immediately look for a liver cell to get into. Penetrating the cell membrane is a formidable task, but the parasites come well-armed for their invasion. Their heads contain a ring of chambers that operate like the barrel of a revolver. When the parasites find a liver cell, they shoot out a blitz of molecules that opens a hole in the cell membrane. These protozoa can’t swim, but they are equipped with little hooks that they use to grab the sides of the hole and pull themselves through. As they’re doing this, the chambers on their heads shoot off another volley of molecules that clump into a protective shroud around the parasites, giving them cover as they work their way inside. When the parasites are completely in, the host cell’s resilient meshwork conveniently—for the parasites, that is—seals the hole shut. The entire operation takes about 15 seconds. It is a breathtaking military operation.17
Once inside the cell, the parasites start multiplying—eventually producing 40,000 or so offspring, called merozoites. The merozoites then spill out of the liver into the host’s bloodstream to begin doing serious damage. But they face a problem: How can they escape the liver cell without getting killed by antibodies? The solution is a disguise. The parasite pulls off the membrane of the infected cell with its little claws and wraps the membrane around itself. Safely disguised as a wandering liver cell, the parasite can wander through the host’s bloodstream to find new targets. Imagine this. It’s like a B-movie prison break where the escapee mugs a guard and steals his uniform to make his getaway. When the parasites cut loose, however, they steal the guard’s entire skin.
In the bloodstream, the protozoa pick out a healthy red blood cell to attack. Their military forces fire off another 15-second barrage of molecules as the parasite claws its way into its new home. Once safely inside, they strip off the liver cell costume and comfortably construct their lethal malaria factory. They begin drinking up the hemoglobin that fills the cell and, fortified with this precious nutrition, rapidly multiply. The growing horde of parasites eventually consumes the entire contents of the red blood cell, which turns into nothing more than a bundle of parasites surrounded by the original cell membrane. When there is no more hemoglobin to drink, the parasites, wrapped in the host’s membrane, move through the bloodstream until they find another healthy blood cell to invade. The process continues from one red blood cell to another, accelerating its pace as the number of parasites exponentially increases at each new stop. Blood cells eventually become infected en masse, and the host falls ill with malaria.
Our immune system is supposed to be our foremost judge and jury for deciphering what is us and what is outsider. This remarkably complex and sophisticated biological system has been honed over the course of evolution to ensure that we and our species endure. It’s a Darwinian masterpiece, homeland security at its finest. If this biological system is so easily misled, is it any wonder we get confused about personal boundaries on the far more subjective level of social behavior?
One might argue that mimics like the parasites that cause malaria are just a band of thieves. Their only interest in our bodies is to steal what they can use. Molecular mimics are simply more sophisticated at being sneaky. Just because they live inside our skin doesn’t mean they are now us. This argument gets shakier when the parasite lives inside our actual cells but, still, one might say, a bloodsucker is a bloodsucker.
What, however, if the visitors didn’t simply destroy you but fundamentally changed you? Parasites like those that cause malaria are akin to irresponsible hotel guests who could care less about trashing their suite. But there are other intracellular invaders who move in with the full intention of staying for the long run. These visitors act more like home buyers. Like most new buyers, they like to personalize the new place to suit their needs. There are, in fact, plenty of parasites like these who completely renovate their new quarters. For example, the larva of the parasite that causes trichinosis, a type of roundworm infection caused by eating raw or undercooked pork or wild game, move inside the host’s cell and, over the next three weeks, first demolish and then reconstruct almost its entire interior: The trichina tear down existing filaments. They even rebuild the roads in and out by modifying the capillaries that control the blood flow to the cell. And the final takeover: They grow additional nuclei. They actually grow new nuclei.18 The host’s tissue is just a pile of lumber as far as these architects are concerned. The trichina don’t just hide inside the host. They transform the host’s insides.
My own nomination for the weirdest biological crossover is Cymothoa exigua, also known as “the tongue-eating louse.” This frightening parasite destroys an entire organ in its host and then replaces it with itself. It invades the mouth of a fish, devours the fish’s tongue and then squats down and neatly positions itself where the tongue was, attaching itself to the tongue’s exposed muscles. The new tongue—the louse—does everything the old one did. It feeds its host just as in the pre-invasion days, gripping and eating prey like a normal tongue. So, which is the host and which is the guest?19
The louse lives off the fish’s blood and other fluids. It’s—trust me—beyond nauseating to look at. When the fish opens its mouth, in place of a tongue you see a slimy, multilegged creature, its beady eyes staring straight at you, its creepy claws reaching out to grab you or anything else that looks like food. But ugly as it may look, the louse doesn’t appear to cause any significant damage to its host. In fact, it keeps the fish alive.
Imagine (apologies for the image) this hijacked fish were you. I’m sure you’d agree that the tongue you were born with is part of your self. But if a tongue-eating parasite moved in, you’d be acutely aware there was a foreign creature living in your mouth. Why, though, should you consider the parasite-tongue to be any less a part of yourself than your original tongue used to be? Is it because your new tongue once lived outside you, or that it has its own genes? Functionally, after all, it’s the same arrangement as before: Both your old and new tongues take in food that keeps you alive, which in turn keeps your tongue alive. What if you had a disease that required your original tongue to be surgically removed? Once it was outside your body, lying pathetically across the room on a table, would you still consider it part of you? Probably not, or maybe you would say it used to be part of you. What if the doctors now fixed the tongue and sewed it successfully back in place? You would probably say it is part of you again, wouldn’t you? So why is the parasite tongue forever an outsider? Just because it happens to have legs and eyes?
Parasites in Paradise
“The sweller”
What: Thread-like nematode called the filarial worm
Where: Tropical and subtropical areas of Southeast Asia, South America, Africa.
How: Larvae inside mosquitos are transmitted via bite to human flesh, where they hatch in lymphatic and subcutaneous tissues.
Outcome: Elephantiasis, or gross enlargement, of the limbs and genitals. Affects 170 million people worldwide.
“The eye worm”
What: Loa loa, a filarial nematode, or roundworm
Where: Native to Ethiopia, inhabits rainforests in West Africa and equatorial Sudan. Loa loa have also been found in India.
How: Infected flies bite humans, transferring larvae onto our skin, which
then travel into our bloodstream and subcutaneous tissue via the bite wound. They have been found in spinal fluid, urine, peripheral blood, and lungs.
Outcome: Most infected people don’t show symptoms. Some may have
itchy, non-painful swellings across the body, muscle and joint pain, and fatigue. Occasionally the loa loa enters the eyeball—when this happens, it can cause severe pain, inflammation, and blindness.
“The pee climber”
What: Vandellia cirrhosa, a small, thin, translucent and sharp-toothed catfish.
Where: Exclusively in the upper Amazon River and Orinoco River basins in northern South America. Usually buried in riverbeds, emerging only to feed or mate.
How: Numerous people report instances of Vandellia cirrhosa climbing up their urethras after they urinated in rivers, though no doctors have been able to corroborate this.
Outcome: The experience is painful, but short-lived, as the fish tend to die pretty quickly after entering our bodies.
At what point do self and non-self merge? What if your own cells were built by an outsider and customized to house that outsider—who would be the tenant and who would be the landlord? Who makes that call? Richard Feynman once said about quantum mechanics, “If you think you understand quantum theory, you don’t understand quantum theory” If you’re not, by now, as confused as I am by what biology teaches us about the notion of a self, I offer one more organism: The mitochondrion.
Parasites are predators. It’s a one-sided relationship whereby the parasite wins and the host loses. At first glance, the tongue-eating louse fits this definition. After all, the little critter invades its victim and bites off its tongue. But, soon after, the louse and its host enter into a remarkable collaboration, what biologists refer to as a mutualistic relationship. Unlike Jerry Coyne’s parasite, the louse is there to stay. It had better, because separation would spell starvation for both parties. Mutualism—give-and-take relationships with outside organisms—are equally essential to human survival. Every one of our cells is a microcommunity, each populated by multitudes of hard-working organelles with their own DNA. Without their work, we wouldn’t last a second. Perhaps the best example of these collaborators are the mitochondria.
Mitochondria are the tiny organelles that generate the ATP molecules that provide the cell with energy. The mitochondria are the cell’s power plant. There are lots of them, anywhere from a few hundred in cells that don’t use much energy to thousands in energy hogs like the cells in our liver, muscles, and brain. It is mutualism at its best. Our life depends on them. They in turn are provided with a safe haven and a steady source of nutrients. The two of us exist as one. Or so it seems.
The mitochondria are so ubiquitous and embedded in our cells’ functioning that it’s easy to forget they’re not technically us. Biologically speaking, the mitochondria are independent organisms. Eons before the formation of complex organisms like our own, they foraged through nature as free-living bacteria. Nowadays the mitochondria live a more domesticated life, commingling safely within the comfort of our cells. But they remain, in the last analysis, not really us: The mitochondria are the only organelles in animal cells that carry their own genetic material, their own DNA (mDNA).20 Mitochondria can grow, replicate, divide, and fuse independently of the cell surrounding them. And our own cells are incapable of manufacturing the mitochondria. Sure, they work for our welfare and we work for theirs, but where it counts—the perpetuation of one’s DNA—they are autonomous agents. “They are much less closely related to me than to each other and to the free-living bacteria under the hill,” observes the biologist Lewis Thomas. “There they are, moving about in my cytoplasm, breathing for my own flesh, but strangers.” The mitochondria are, by definition, others.
Sort of, that is. “I was raised in the belief that these (mitochondria) were obscure little engines inside my cells, owned and operated by me or my cellular delegates, private, sub-microscopic bits of my intelligent flesh,” Thomas remarks. But, as he learned more about these organelles, when he understood just how much claim they had to their own individuality, he was forced to alter his view of his own self: “Looked at this way, I could be taken for a very large, motile colony of respiring bacteria, operating a complex system of nuclei, microtubules, and neurons for the pleasure and sustenance of their families.”
Organisms living within organisms are nothing special in the world of microbiology. If you look inside many house plants, for example, you’ll find a little insect called a mealybug carrying out a life of its own. Look inside the mealybug and you’ll find collaborations of bacteria living their own lives. And inside these bacteria are smaller, separate bacteria.21 And there are plenty of other outsiders. The mitochondria, like the mealybugs, mock the notion of a self. Squint into a microscope, and these organelles within organelles look like independent creatures, each going about its own work. Step back, however, and the mitochondria appear to simply be one of many elements that compose the single complex unit we call a cell.
Are the mitochondria us or them? It doesn’t much matter how we answer this question. The fact is there are genetically independent organisms living, seamlessly enmeshed, throughout our bodies. Selves within selves. We’re like those Russian matryoshka dolls that open to reveal a succession of progressively smaller dolls inside. There are the little mitochondria dolls inside the bigger mitochondria dolls inside the biggest doll of all—us. But, hold on, how can we be sure there’s not a bigger doll yet? It’s a tough question.
Is there anything in the mix we’re left to call our own? Lewis Thomas leaves us with a modest plea: “I only hope I can retain title to my nuclei.”22 It will be interesting, as microbiologists dig deeper into our structure, to see whether even this turns out to be true. For starters, what about those trichinosis parasites who enter our cells and grow their own nuclei?
Where does one draw the line between self and other? In biology, the answer boils down to genetics. A self, as Richard Dawkins famously argued in his classic book The Selfish Gene, is nothing more than a set of DNA intent on replicating itself. Different DNA, different self. By this definition, my mitochondria are individuals separate from me. Yet, even to geneticists, my mitochondria’s genetic individuality represents the essence of my own individuality, not simply who I am today but who I descend from. How can my ancestral fingerprint not be me?
But if the mitochondria are me, doesn’t this mean I have two sets of genes? Aren’t I a mosaic of both my own cellular DNA and that of my mitochondria? The fact is that all of the “others”—whether they are parasitic or mutualistic, cheaters or straight-shooters, long-term residents or one-night stands—have a significant characteristic in common: They each carry their own DNA. And this means that, for however long they are inside their host’s body, two genetically distinct organisms are living under the same skin and, to one extent or another, are biologically intertwined. Deep down, at the core of our tissue, we are a gigantic, symbiotic array, a ragtag assortment of organisms. All of these are to some degree us.
Then again, results are more important than who gets credit for what. The fact is that somehow—miraculously really—when the pieces are tossed together, the machine we call our body actually works. And the reason it does has less to do with competing than cooperating. As evolutionary biologists Lynn Margulis and Dorion Sagan point out, “Life did not take over the globe by combat, but by networking.”23
Robert V. Levine is a professor of psychology at California State University, Fresno and former president of the Western Psychological Association. His previous books include A Geography of Time and The Power of Persuasion: How We’re Bought and Sold.
References1
1. Coyne quoted in Forsyth, A. & Miyata, K. Tropical Nature Charles Scribner’s, New York, NY (1984).
2. Krulwich, R. & Abumrad, J. producers and co-hosts, Radiolab (WNYC/NPR); interview with Jerry Coyne, Dec. 12, 2008.
3. Jerry Coyne, personal communication, Feb. 16, 2010.
4. Coyne, interview by Krulwich.
5. Ibid
6. Ibid
7. Ibid
8. Jerry Coyne, personal communication, Feb. 16, 2010.
9. Coyne, interview by Krulwich.
10. Ibid
11. Ibid
12. Jerry Coyne, personal communication, February 16, 2010.
13. Coyne, interview by Krulwich.
14. Jerry Coyne, personal communication, Feb. 16, 2010.
15. Paul Crosbie, personal communication, Feb. 26, 2010.
16. Darwin quoted in Zimmer, C. Parasite Rex Free Press, New York, NY (2001).
17. Zimmer, Parasite Rex.
18. Ibid
19. Brusca, R. & Gilligan, M.R. Tongue replacement in a marine fish (Lutjanus guttatus) by a parasitic isopod (Crustacea: Isopoda). Copeia 3, 813–816 (1983).
20. Lane, N. Power, Sex, Suicide: Mitochondria and the Meaning of Life Oxford University Press, New York, NY (2005). See also Saey, T.H. Repairing a cell’s faulty batteries. Science News 177, 16 (2010).
21. Lane, Power, Sex, Suicide, 111–14.
22. Thomas, “Organelles as Organisms.”
23. Margulis, L. & Sagan, D. “Rethinking life on Earth: The Parts; Power to the Protoctists,” Earthwatch 11, 25–29 (1992).
An excerpt from an unpublished book that Robert V. Levine is currently working on.