This was the moment Hilde Proescholdt had been waiting for. Peering through her bench-top microscope in 1921, she beheld the soft mass of a salamander embryo. Fertilized two days earlier, the developing creature, no larger than a grain of sand, was now a hollow sphere of thousands of mostly uniform cells. One clump, however, had started to migrate inward, toward the center of the sphere, and Proescholdt saw that it had formed a dimple, like the impression left by a finger pressed into an inflated balloon.
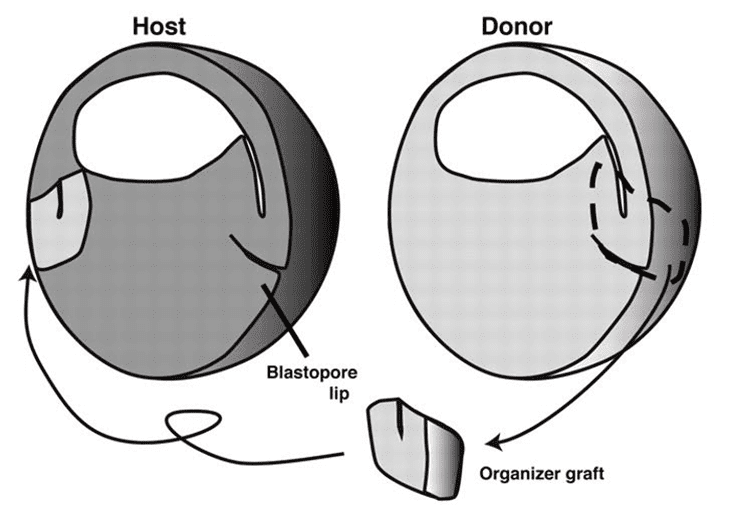
Delicately, as a surgeon wields a scalpel, she guided a fine glass needle through the embryo, carving away the dimple, known as the dorsal lip. With a micropipette, she added the excised cells to a young embryo of a different salamander species, at a site opposite its own dorsal lip, such that the two lips framed the tiny ball like a pair of ears. Then she gently pressed the cells—transplant and host—together underneath a glass slide, submerged them in pond water, and waited for them to grow, hoping her chimeric creation would unlock one of the greatest mysteries of her time: How does an embryo become an animal?
The dorsal lip provided the instructions for development, dictating the destinies of the cells surrounding it.
Dissections in the early 1800s had confirmed that a body grows from a single fertilized egg, or zygote—not from a miniature “preformed” being, as some scientists believed. But what orchestrates this transformation? As the zygote divides, churning out virtually identical cells, each clone must figure out which tissue or organ it will ultimately become. Will it morph into skin? Intestines? Brain matter? Somehow during the metamorphosis from sphere to salamander (or sparrow, or sperm whale), an embryonic cell must learn its adult identity.
Proescholdt was about to find its teacher.
The daughter of wealthy merchants, she had arrived at the University of Freiburg the previous year to pursue a Ph.D. under the embryologist Hans Spemann. These were turbulent times in Germany. The end of World War I, in 1918, had spurred political unrest, along with inflation and food rations. One winter, students survived almost entirely on turnips.
Proesholdt found solace in nature (the campus abutted the Black Forest) and intellectual discourse. A lively conversationalist, she spent long evenings debating literature, philosophy, art, and science. “We cared more about food for thought than about nourishment of our bodies,” her friend and bench mate Viktor Hamburger later wrote.
She was disheartened, then, when her first project in Spemann’s lab turned out to be tediously dull. While her male peers pursued problems related to Spemann’s groundbreaking work on early embryo development, he had her replicate an obscure, decades-old experiment. In the late 1700s, the French naturalist Abraham Trembley claimed that when he flipped a hydra—a tiny Medusa-like invertebrate—inside-out, its insides morphed into its outsides, and vice versa. Proescholdt’s assignment was to verify that this was true.
“There was no doubt about Spemann’s prejudice against women,” wrote geneticist Salome Waelsch, in 1992. When Waelsch had joined his lab in 1928 as a graduate student, he had similarly tasked her with a “boring descriptive study … which would provide the essential basis for [a] young man’s quite exciting experimental problem.”
Unable to duplicate Trembley’s results, Proescholdt became increasingly frustrated. At last, after he too failed to get the hydra to cooperate, Spemann set her to work on one of his highest-priority experiments.
Scientists had long suspected that some cells could tell other cells what to become.
It was the culmination of nearly two decades of study. In the early 1900s, Spemann had found that he could manipulate the growth of a young embryo by constricting its spherical mass with a tiny lasso made from human hair, which he had cut from his infant daughter’s head. Her hair was so fine that Spemann could split an embryo in half just by tightening the lasso. And in doing so, he had observed two possible outcomes.
When the lasso cut through the part of the embryo that gives rise to the dorsal lip, giving each half an equal portion, two salamanders had developed—identical twins. In all other cases, only the half containing most or all of the dorsal lip had grown into an adult, while the other half had become a clump of blood and guts, which Spemann dubbed the “belly piece.”
He had concluded that the dorsal lip must be responsible for an embryo’s development. At first, he had thought that this tissue simply grew into the defining features of a salamander, following instructions from some unknown cellular program. Then in 1919, he had received a letter from a colleague, who had proposed an alternative explanation: The dorsal lip itself provided the instructions for development, dictating the destinies of the cells surrounding it. A decade earlier, in 1909, embryologist Ethel Browne, while a graduate student at Columbia University, had shown that a small group of cells in a hydra organized its form. Perhaps vertebrate embryos grew by similar means.
It was this theory that Proeschold set out to test in 1921. By grafting the dorsal lip from one species (whose cells were white) onto the embryo of another species (whose cells were brown), she could track the fates of these differently colored tissues. Would the white-celled dorsal lip grow into the features of a salamander, as Spemann had originally surmised? Or would it recruit the brown cells of its host to do its bidding, as his colleague had argued and Browne’s study suggested?
The work was painstaking. Wielding rudimentary tools, and without antibiotics to stave off infection, Proescholdt watched as graft after graft failed to take or the conjoined cells perished. Then one day in May, an embryo survived. In the three days since its operation, the tiny sphere—pocked by two dorsal lips at opposite poles (one white, the other brown)—had grown into a pair of Siamese twin tadpoles joined at the belly.
Under her microscope, Proescholdt could see that the transplanted dorsal lip (white cells) had formed only some muscles and other supportive tissue of the nearest twin. The rest of its body—head, brain, spinal cord, kidneys—came from the host embryo (brown cells). Spemann’s colleague had been right: The dorsal lip was the embryo’s foreman. Spemann named it “the organizer.”
It took Proescholdt 259 trials over two years to repeat this result five times, enough to warrant publication, in 1924. To her annoyance, Spemann insisted on adding himself as first author on the paper, even though his male students enjoyed solo bylines. He would later win the Nobel Prize for the discovery; Proescholdt would be all but forgotten for more than 60 years.

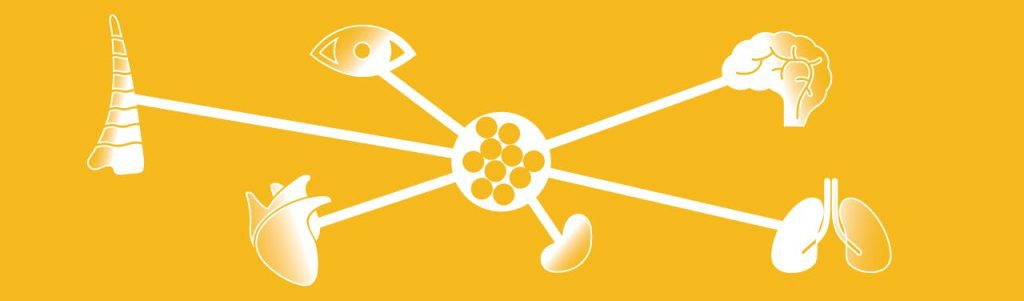
The organizer study, meanwhile, became famous—and then infamous. Scientists had long suspected that some cells could tell other cells what to become—a process known as induction. The cells in a developing frog’s eye, for example, will enlist surrounding skin tissue to build an optical lens. But Proescholdt, under Spemann’s advisement, had found the epitome of induction in the dorsal lip. Here was a Lilliputian population of cells that could direct the formation of an animal, including the most baroque system ever evolved: the central nervous system.
But how did it work? If the organizer gave embryonic cells their marching orders, what language did it speak? Biologists began hunting for the organizer’s molecular messenger: a chemical that could explain its dictatorial influence. The search party spanned the globe. The amphibian embryo, wrote American biologist Ross Granville Harrison, had become the “new Yukon to which eager miners were now rushing to dig for gold.”
Time and time again, however, the diggers came up dry. “[The organizer] maintained this mysterious status,” says Richard Harland, a geneticist at the University of California, Berkeley. “It was one of these problems in biology that people were very puzzled by.” Further confounding matters, researchers learned that a disabled or dead organizer, although it couldn’t build a whole organism, could still induce neural tissue from embryonic cells. Even an unrelated substance—sand, clay, turpentine, boiled guinea pig liver, or calf testicle—would do the trick.
Scientists now know that salamander tissue, for mysterious reasons, is particularly sensitive to any outside disturbance, which is partly why embryologists have moved on to other model organisms such as the African clawed frog. But no one knew this in Spemann’s time. By the late 1930s, many of his peers had begun to doubt the organizer’s power. Then World War II broke out, and some of the last of the organizer’s champions in Germany fled.
Eddy De Robertis, an embryologist at the University of California, Los Angeles, remembers learning about Spemann’s work as a graduate student in the 1970s. His professors, he says, told him that Spemann’s obsession with the organizer had set back the field of developmental biology 50 years. “It was considered something totally ridiculous,” he says.
When Spemann died in 1941, at age 72, the organizer nearly did too.
As for Proescholdt, she had married Spemann’s assistant Otto Mangold, taking his name. The young couple had moved with their infant son to Berlin in the spring of 1924, when Hilde was 25. By September, as the organizer paper went to press, she was dead.
Published accounts of her death call it an accident—a kitchen fire sparked by a faulty gas stove. “The truth of the matter is that she killed herself,” says Claudio Stern, a developmental biologist at University College London who had heard the story from Mangold’s (Proescholdt’s) friend and contemporary, Salome Waelsch.
For six decades, Mangold’s legacy stayed buried with her. Then in 1988, her former lab mate Viktor Hamburger, then a celebrated biologist in his 80s, published a memoir about his time in Spemann’s lab and his friendship with Mangold. “Very few of her contemporaries are still alive,” he wrote. “As one of them who knew her well, I feel I should rescue her from oblivion.”
“A lot of people read that book—it was fascinating,” says De Robertis. He credits Hamburger with resurrecting not just Mangold’s name but also scientists’ interest in the organizer. Spemann and his contemporaries had failed to find its elusive chemical signal. But now, armed with modern genetic tools, De Robertis and his colleagues thought they might have better luck.
His lab made the first breakthrough in 1990. “I was so happy that I jumped on top of the bench,” De Robertis says. His team had homed in on a specific gene, called goosecoid, that was active only in the organizer, meaning the gene produced proteins that might be part of the organizer’s chemical toolkit. To find out, De Robertis’ student Ken Cho had injected goosecoid copies into a frog embryo, opposite its dorsal lip—at the very spot where Proescholdt had placed her tissue graft. Three days later, Cho and De Robertis were looking at conjoined twin tadpoles. No longer was the organizer just a mysterious piece of tissue—it had a molecular identity.
But goosecoid doesn’t work alone. In the past two decades, De Robertis and other researchers have unveiled a network of organizer genes that together produce a cocktail of signaling proteins which diffuse across an embryo in varying concentrations. “You have this gradient along which cells are moving and getting various instructions,” says De Robertis. “We call it positional information.” The strength of the signal determines which genes in a cell’s nucleus get switched on or off, thus telling the cell which type of tissue or body part it will become.
Scientists are still working to decode this chemical dialogue. What’s clear, however, is that the animal kingdom wouldn’t exist without it: Neither a salamander embryo nor human fetus would amount to much more than bloody “belly piece.” If you silence the organizer, as De Robertis says, “you lose everything.”
Alex Riley is a freelance science and nature writer based in London. His work has been published by Aeon, NOVA Next, the BBC, and other outlets. Follow him on Twitter at @riley__alex.
*This image was reprinted from Harland, R. Induction into the Hall of Fame: Tracing the lineage of Spemann’s organizer. Development 135, 3321-3323 (2008).