As they often do after a rainstorm, butterflies had gathered around puddles on Pigeon Mountain in northwest Georgia. Nets in hand, James Adams and his friend Irving Finkelstein watched the insects lapping up salts and proteins dissolved in the muddy water, their folded wings yawning apart now and then. There were silvery-blue Celastrinas and Skippers the color of cinnamon and ash. Largest of all were the Tiger Swallowtails—pastel lemon males with black dagger-like stripes and midnight-dark females with a dusting of evening cerulean.
Suddenly a very odd creature flitted past Adams and Finkelstein—a swallowtail unlike any they had ever seen. Its left half was yellow; its right, black. It was as though someone had sliced up two different insects and seamlessly sewn them back together. Finkelstein yelped and took a swipe at the bizarre beauty, missing by quite a bit. Suppressing his excitement, lest it misguide his hand, Adams chased the butterfly a few steps, swung, and netted it. He could see immediately that he had caught a gynandromorph—an animal that was half-male and half-female.
Butterfly collectors love gynandromorphs for their rarity as much as their peculiarity. They are unpredictable hiccups in nature’s symphony of symmetry. The creatures tantalize scientists, too, because they offer a unique opportunity: the chance to study typically male and female genes and anatomy in the same body.
For hundreds of years, naturalists have been documenting gynandromorphs among insects, spiders, lobsters, and birds. More recently, researchers—aided by increasingly sophisticated laboratory tools—have overturned reigning theories of sexual development by studying such hybrids. As has proven true time and again throughout the history of science, the creatures that seem strangest—those that are too odd, too asymmetrical to fit neatly into our presupposed categories—teach us the most about how all living things work. It turns out, for example, that the standard explanation of how a bird becomes male or female is wrong. Scientists came to this realization not by investigating scores of typical birds, but rather by examining a few gynandromorphs. It all started with an odd zebra finch.

In the late 1990s, a lab animal caretaker at Rockefeller University kept finding eggs in a cage that supposedly contained only male zebra finches. To identify the culprit, she moved all the birds into individual enclosures. The eggs were coming from a bird that, as it grew older, looked increasingly different from the rest. On its right side it had all the typical features of a male: a blush of orange plumage on its cheek, a zebra-striped neck, and a handsome patch of brown feathers speckled with white dots near its wing. In contrast, its left side was almost entirely gray with a few black and white facial markings and an egg cream breast—characteristically female plumage. Realizing that this bird was a gynandromorph, Fernando Nottebohm, the Rockefeller neuroscientist who owned the finch colony, gave the bird to his former student, Art Arnold, at the University of California, Los Angeles. He suspected that Arnold, who studied gender differences, might learn something from the creature.
Scientists had long assumed that birds become male or female the same way mammals do: They start out as unsexed embryos that, depending on the sex chromosomes, grow either a pair of testes or ovaries, which in turn release wave after wave of hormones to masculinize or feminize the organism. Yet now and then studies had uncovered inklings that this story was too simple.
Following these clues, Arnold and his colleagues decided to take a close look at the cells in the gynandromorph finch’s brain. Typical male zebra finch brains have a network of neural circuits dedicated to learning courtship songs, and the area containing these circuits is much larger than the corresponding region in the female brain. If sexual development was dependent primarily on hormones, then each half of the gynandromorph’s brain should be architecturally identical. After all, every organ in the bird’s body was bathed in the same androgynous cocktail of sex hormones released by its testis and ovary.
Multiple sclerosis, cancer, heart disease, and many other illnesses are much less common, or less deadly, in one sex or the other.
What Arnold and his team discovered, however, was that the singing region was 82 percent larger in the right half of the bird’s brain than in the left. To investigate further, the researchers sliced up the brain and soaked the thin sections in a solution brimming with bits of radioactive RNA designed to bind to either the Z or W sex chromosomes, the avian correlates of our X and Y. Laying the soaked slices onto photographic plates revealed that the brain’s right hemisphere was comprised mainly of male cells with two Z chromosomes, whereas most of the left-hand cells had the female combination of a Z and W. The sex chromosomes, not hormones, had dictated the brain’s fate, cell by cell.1 “That one gynandromorph changed my mind about some very fundamental scientific dogma,” Arnold says. “Brain cells are not a tabula rasa on which hormones write sex. They come pre-described.”
In the mid 2000s, Michael Clinton, a developmental biologist at Edinburgh University, began studying gynandromorph chickens collected from poultry farms around the United Kingdom. On one side, the birds looked like males: white and gold plumage, a big red wattle, and a long hornlike protrusion on the leg known as a spur. On the other side they had female features: brown and russet feathers, and a much smaller spur and wattle. When Clinton and his team analyzed DNA from blood, skin, and muscle cells around the fowls’ bodies, they found that the chickens were not just superficially split. As with the unusual zebra finch brain, their entire bodies were more or less divided on a cellular level, with lots of female ZW cells on one side and mainly ZZ cells on the other—but there was a bit of cellular scrambling, too. They were kind of like bisected jars filled with two different flavors of jellybeans that mostly kept to their own sides but sometimes crossed over.
These chimeras confirmed that a bird’s sex is determined cell by cell throughout the body, rather than uniformly through hormones as in mammals.2 What remains unclear is exactly how the sex chromosomes within each cell shepherd sexual development independent of sex hormones. Perhaps the Z and W chromosomes initiate certain epigenetic changes—that is, changes to the molecules surrounding DNA that increase or inhibit the activity of different genes—from the moment of fertilization. Supporting this hunch, Clinton and his colleagues discovered that typically developing male and female bird embryos have divergent patterns of genetic activity even before sex organs develop.
Arnold and his team now want to know whether what they discovered in birds applies to mammals, too. To tease out the distinct roles of hormones and chromosomes in mammalian sex development, he and his colleagues have been studying unique strains of mice that have, say, one too many X or Y chromosomes or churn out female sex hormones even though they are genetically male. He hopes that this line of investigation will eventually yield new treatments for human maladies. Multiple sclerosis, cancer, heart disease, and many other illnesses are much less common, or less deadly, in one sex or the other. “If we can figure out why one sex is protected we can figure out new therapies,” Arnold says. “To understand that, we need a list of factors that make males and females different. It used to be all about the hormones.”
Multiple sclerosis, for example—a devastating illness in which the immune system attacks and destroys the nervous system—is much more common in women but more rapidly debilitating in men. Arnold’s studies on mice that are genetically male but produce female hormones suggest that something about the combination of an X and Y chromosome—not male hormones—make cells more vulnerable to a severe course of a similar disease.3
The zebra finch and chicken studies astounded researchers beyond the bird community. They “really argued against some longstanding notions we had about how cells get their sexual identity in vertebrates,” says Nipam Patel, a developmental biologist at the University of California, Berkeley. As those experiments were underway, his own research on unusually sexed butterflies was overturning entrenched ideas as well. After more than a decade of obsessive study, Patel has concluded that in some cases, rather than a single animal with male and female parts, a gynandromorph is two different creatures fused together.
When Patel was about 8 years old, he came upon a dead male two-tail swallowtail butterfly lying in the grass in his backyard. He was entranced by its beauty: the way those yellow birdlike wings swooped out from the insect’s body, only to arc in return, ripple and drip into a parenthesis. Patel carried the swallowtail inside and mounted it in a cigar box. He convinced his mother to sew him his first butterfly net. Since then, Patel has amassed a library of around 30,000 insects. And he has spent the past 16 years curating a second collection: not of physical specimens, but rather of photographs. Patel is the world’s foremost collector of gynandromorph butterfly pictures.
Gynandromorphs appeal to Patel because they are naturally occurring experiments that allow him to study how animals develop. Many scientists have attributed the existence of gynandromorph butterflies to a cell division error. Like birds, male butterflies have two Z chromosomes in every cell, whereas female cells are ZW. When a caterpillar begins metamorphosis, the standard story goes, the dividing cell that will eventually produce the wings multiplies its chromosomes as usual, but fails to properly divvy up the sex chromosomes in its two daughter cells. One of the new cells gets a Z and W and becomes female; the other gets one or two Z chromosomes and becomes male. Each of those cells is the progenitor for either the left or right wing, so the butterfly looks half male and half female—split down the middle.
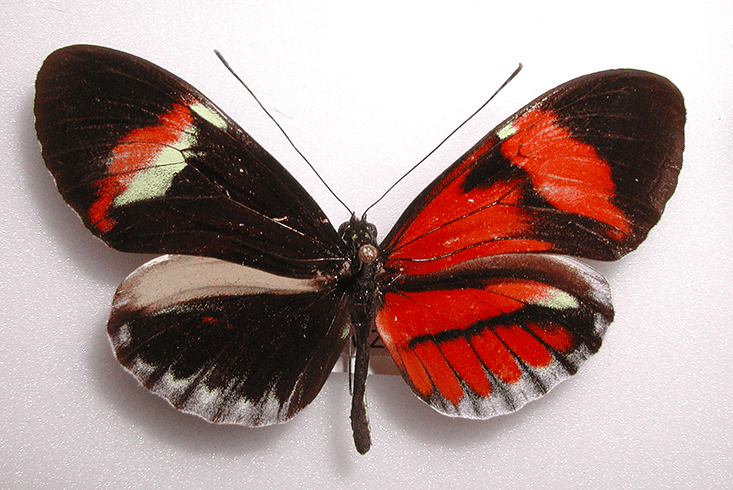
Scientists have clearly shown this to be the case, but some of Patel’s photos have made him think that gynandromorphs form via other routes as well. Every now and then he has encountered specimens such as the Heliconius butterfly pictured above. It’s a member of a colorful tribe in which males and females look nearly identical. Patel identified this butterfly as a gynandromorph because the two halves of its abdomen differed subtly in size and structure, one having male genitalia and the other female. Its wings should have been the same, but they weren’t.
Patel realized that, in addition to being doubly sexed, this particular insect might have a mixed identity. The patterns on its wings betrayed underlying genetics that could not be explained solely by a medley of female and male cells. A gene named optix, for example, determines where red rays grace the hindwings; another gene called WntA acts like a black sharpie that darkens the wing here and there. Neither of these genes lie on the Z or W chromosomes—the only source of genetic variation between a gynandromorph’s two halves, according to the standard origin story.4 Yet the two wings had clearly activated different color pattern genes, indicating two entirely different genomes. When gynandromorphs differ in more than their sex characteristics, Patel thinks you could be looking at two animals in one.
How could that happen? An insect egg cell harbors a smaller sister cell known as a polar body—a leftover from the cell division that created the egg cell. Sometimes two sperm manage to slip inside the egg and fertilize the egg’s nucleus as well as the polar body, creating two embryos that develop more or less like conjoined twins. The two animals’ cells may not always keep to their own sides, however, which could explain why sometimes a gynandromorph has bilateral asymmetry and sometimes ends up a mosaic, with a splattering of opposite cells across a center line. Although other scientists have previously suggested that double fertilization accounts for some gynandromorphs, Patel has uncovered some of the clearest evidence to date.
Gynandromorphs’ duality calls to mind Plato’s Symposium, in which Aristophanes explains the origin of the sexes with a unique creation myth: In the beginning, he says, there were double-bodied primal humans—man and man, woman and woman, and man and woman—until, angered by their hubris, Zeus split them with bolts of lightning. But such duality is not just the stuff of myth.
The more scientists fingerprint the genetic components of living things, the more they realize that we are all multifarious creatures. Every one of our cells essentially has two genomes: the human one in our nucleus and a non-human tucked inside mitochondria that were once free-living microorganisms. Trillions of bacteria cover our skin and dwell inside our intestine, turning our bodies into a kaleidoscope of microbial genomes. And quite a few foreign genes have infiltrated our genome with the help of viruses and various parasites. Gynandromorphs catch our eye because their striking variegation seems so exceptional. In truth, they are mirrors reflecting the tessellations within us all.
Ferris Jabr is a contributing writer for The New York Times Magazine and Scientific American. He has written for The Atlantic, Harper’s, The New Yorker, and Outside. His work has been anthologized by The Best American Science and Nature Writing series.
References
1. Agate, R.J., et al. Neural, not gonadal, origin of brain sex differences in a gynandromorph finch. Proceedings of the National Academy of Sciences 100, 4873-4878 (2003).
2. Zhao, D., et al. Somatic sex identity is cell autonomous in the chicken. Nature 464, 237-242 (2010).
3. Du, S., Itoh, N., Askarinam, S., Hill, H., Arnold, A.P., & Voskuhl, R.R. XY sex chromosome complement, compared with XX, in the CNS confers greater neurodegeneration during experimental autoimmune encephalomyelitis. Proceedings of the National Academy of Sciences 111, 2806-2811 (2014).
4. Joron, et al. A conserved supergene locus controls colour pattern diversity in Heliconius butterflies. PLOS Biology 4, e303 (2006).
Lead image is a photograph of Mocker Swallowtail (Papilio dardanus) butterflies, with a female to the left, gynandromorph in the middle, and male on the right. Oxford University Museum of Natural History.
This article was originally published in our “Symmetry” issue in May, 2014.