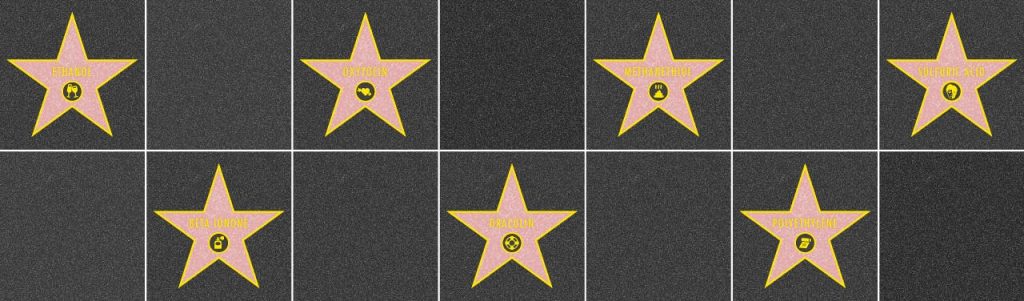
From drinking water to DNA, from caffeine to carbon dioxide, and from Lipitor to Viagra—that is from atorvastatin to sildenafil citrate—molecules define our personalities, regulate our abilities, and dictate our feelings. Invisible to the human eye, many of them are biological celebrities: They famously smell or stink, make us feel depressed or elated, pollute our planet or save our lives. Even the most destructive molecules are so essential to our civilization that modern industry wouldn’t exist without them. Here we describe seven of the most prominent corpuscular figures, without which our life would be completely different. In fact, without some of them, scientists say, there’d be no human life at all.
Beta-ionone—the sexy scent superstar
Beta-ionone, the sweet-smelling molecule that gives many a flower its pleasant alluring aroma, is famed for its sexy odor. You smell it when you sniff wild violets, enjoy your Valentine’s Day bouquet, or catch a whiff of Chanel perfume.
“Flowers are the sexual organs of plants,” says Ayala Moriel of Ayala Moriel Parfums, which specializes in natural scents. Beta-ionone is important to flowers’ reproductive strategy and helps attract bees and other insect pollinators.
But it also happens to be pleasing to humans, which explains its celebratory status in the world of manufactured scents. Chanel No.19, Fleur No. 1, Après l’Ondée, l’Heure Bleue, and many others use beta-ionone.
Beta-ionone is highly volatile, meaning it vaporizes easily—otherwise it would never waft off the blossoms. It also has an extremely low odor-detection threshold. While its molecular formula is always the same, CHO, the experience of smell varies greatly from person to person, Moriel says. “Beta-ionone smells powdery but with a green scent, although some might find it more fruity, like raspberry.”
Ethanol—the celebrated intoxicant
From punk rock shows to operas and from weddings to world leaders’ meetings, ethanol, more commonly known as alcohol, CHO, is poured into every celebratory champagne flute, chilled martini glass, and frothing beer mug.
Quickly absorbed by the blood stream, ethanol molecules travel to the liver, kidneys, and also the brain, where they interfere with the neurotransmitter receptor proteins involved in memory, speech, and decision-making. These receptors contain cavities that are normally filled with water, but ethanol works its way into the cavities, displacing the water.
“In most proteins this displacement wouldn’t make any difference—ethanol is close enough to water that the protein doesn’t care,” said R. Adron Harris, Director of the Waggoner Center for Alcohol and Addiction Research at the University of Texas, Austin. “But in certain proteins in the brain, ethanol changes their function.”
Ethanol stimulates gamma-aminobutyric acid (GABA) receptors, which have a brain-dampening effect, and inhibits glutamate receptors, which have a brain-stimulating effect. Ethanol also stimulates the production of happiness-inducing brain chemicals, including dopamine and endorphins. The result is the slurry, blurry good cheer we know as intoxication.
Because a single gram of alcohol increases urination by 10 mL, drinkers become dehydrated. As the body metabolizes ethanol, water reinserts itself into protein cavities, and then the brain finds itself in overdrive. The result is the headaches, sleep disruption, fatigue, and nausea of a hangover. That’s why many party-goers find that a drink the next morning alleviates the symptoms and why chronic alcoholics need a reinfusion so desperately.
Oxytocin—famed for love and other good deeds
Alcohol is nothing compared to the intoxicating power of oxytocin, dubbed in popular literature as the love hormone. “There would be no human beings without oxytocin,” says behavioral endocrinologist Sue Carter, who has been studying the molecule for 33 years.
Studies link oxytocin to orgasmic pleasure, protective feelings toward those we love, stress relief, satiety, generosity, and even certain kinds of memory recall. Oxytocin causes uterine contractions during labor. While a number of mammals deliver their offspring without it, human babies, with their large heads, need its help to be pushed through the mother’s cervix. It also triggers lactation in nursing moms. “It’s an ancient molecule,” Carter says. “It originated long ago, and has been reused over and over again by our thrifty biology for multiple purposes.”
“There would be no human beings without oxytocin,” says behavioral endocrinologist Sue Carter.
Oxytocin is composed of nine amino acids containing the standard organic chemistry building blocks—carbon, hydrogen, nitrogen, and oxygen—but, fittingly for a love molecule, it also has two devilish sulfur atoms (CHNOS).
“Disulfide bonds are the most labile bonds in biological systems, meaning that they break easily,” says Lance Martin, founder of Martin-Protean, a company that investigates the molecular properties of oxytocin. Martin says that oxytocin courses through our system in a dormant form—its disulfides bond with some non-reactive protein—until the biomolecular context changes and some new proteins, hormones, or other elements enter the scene. The disulfide bonds break easily and oxytocin gets to work—relieving stress, inducing happiness, and prompting feelings of trust, among other things.
Draculin—the potential lifesaver
Sanguinivorous bats use Draculin, a powerful anticoagulant made up of more than 400 amino acids, to keep their prey’s blood flowing during feeding. And that’s exactly the reason why recent clinical studies suggest the molecule can help treat stroke victims; it can dissolve blood clots.
Of the nearly 800,000 strokes that befall Americans each year, about 137,000 are fatal. About 87 percent of strokes are ischemic, meaning a blood clot cuts off the brain’s oxygen supply. The current standard of care, a protein called a tissue plasminogen activator (tPA), can dissolve such clots, but may cause brain damage. In most cases, tPA must be administered within three hours of the stroke—any later and the risks outweigh the potential benefits. Draculin could extend that window to as much as nine hours. And unlike tPA, draculin has not shown negative effects on the brain. It focuses instead on an enzyme called Factor X, inhibiting its role in blood-clotting.
Polyethylene—the renowned pollutant
Polyethylene takes years to decompose—even left out in the sun it’s not going to biodegrade in our lifetime. It is a polymer consisting of a string of methylene units with two terminal hydrogen atoms that balance the chemistry. But each methylene unit1 is nothing but one carbon atom and two hydrogens; so on a molecular level, a plastic bag would be as organic as the tree branch it dangles from.
In the coming decades, it may well be that polyethylene bags will grow on trees rather than hang off the branches.
“Polyethylene has a degradability problem on several levels because it is so simple,” says Regina Valluzzi, who has studied polymers for two decades. “To get a chemical reaction you need some spot on the molecule that’s reactive—an acid or base, an electron-sucking group, double bonds, a ‘shaped’ area of chemical interactions for enzymatic attack. Polyethylene does not offer good reactive sites.”
Polyethylene’s stability is only one reason that it is so popular. In its densest forms, it is stronger than Kevlar. Its high hydrogen content makes it a powerful barrier against certain forms of radiation.
While polyethylene is typically derived from petroleum or natural gas, it can also be produced using organic materials. While shoppers weigh the merits of paper vs. plastic, scientists are contemplating oil vs. biomass, so in the coming decades, it may well be that polyethylene bags will grow on trees rather than hang off the branches.
Methanethiol—the famous stinker
Farts. Rotten cabbage. Bad breath. Decaying flesh. You know methanethiol when you smell it—or expel it. Four hydrogens and a carbon are joined by an atom of sulfur to create one stinkbomb of a molecule, CHS, which is also released when we metabolize asparagus, creating the legendary phenomenon of “asparagus pee.” They can also be found in skunk spray and hours-old human sweat. The methanethiol molecule looks similar to the ethanol one, except there is one more methylene group and the
oxygen is replaced by sulfur, which is why instead of getting you drunk, it smells terrible. So terrible, that the human nose can detect one methanethiol molecule in a billion other molecules. They send a very clear olfactory message: Keep your distance.
That’s what the stinker is famed for—its offensive odor keeps us away from spoiled food and decomposing feces. It also saves lives. It is added to natural gas, which is colorless, odorless, deadly to breathe, and highly combustible. Methanethiol infusion gives this hard-to-detect chemical a smell—or rather an impossible to ignore stench, a clarion call to fix the leak or run to safety.
Sulfuric Acid—infamous villain and beautiful hero
When a mobster threw sulfuric acid at Gotham City district attorney Harvey Dent, it destroyed half his face and seared away his sense of right and wrong, transforming him into Two-Face, a well-known foe in Batman’s gallery of rogues.
Nicknamed oil of vitriol, sulfuric acid, HSO—a colorless, odorless, oily liquid—eats metal and stone, expelling potentially explosive hydrogen gas. In addition to its acidity, it has a deranged appetite for water, sucking the moisture out of wood, paper,
cotton, and human flesh so quickly that these materials char from dehydration. It swells and burns flesh on contact, often leaving scars that never disappear.
“I love sulfuric acid,” says chemistry professor and Nobel Laureate Roald Hoffmann.
Yet, we need this ill-famed destroyer on a daily basis. Sulfuric acid is used to make fertilizers and explosives. It’s the acid in car batteries. It removes impurities from metal surfaces. It’s crucial to producing rayon and other semi-synthetic fibers. Cleansers, wastewater processing, oil refining, and industrial chemical manufacturing all depend on the very same noxious properties of sulfuric acid. The US produces more than 40 million tons of sulfuric acid every year—more than any other manufactured chemical.
“It is the ultimate transformer of other molecules, even if you can’t buy it in a supermarket,” says chemistry professor and Nobel Laureate Roald Hoffmann. In the eyes of a chemist, this molecule is so immensely useful, he calls it beautiful. “I love sulfuric acid,” he says.
Patchen Barss is a Toronto-based journalist specializing in new ideas in science and the humanities. He is the author of The Erotic Engine: How Pornography Powered Mass Communication from Gutenberg to Google, and has written for many publications, including Saturday Night, the National Post and the Montreal Gazette.