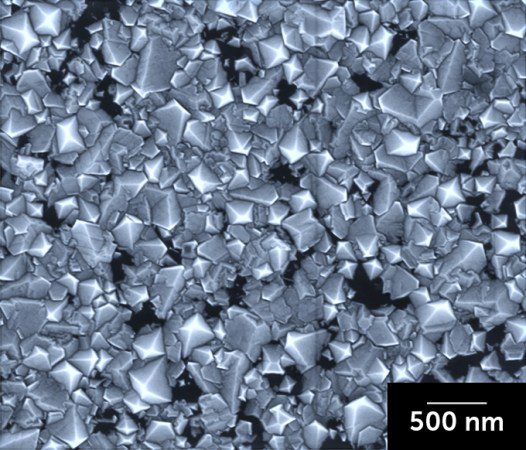
Diamonds normally form under very high pressure in the Earth's mantle. But a new method developed in laboratories allows diamonds to form without this intense pressure.
The most common way to make synthetic diamonds is through high-pressure and high-temperature growth, which needs about 5 gigapascals of pressure, similar to the conditions in the upper mantle where diamonds naturally form. Using this technique, carbon dissolved in liquid metal creates diamonds at temperatures around 1400° Celsius.
But diamonds can be grown at atmospheric pressure in a liquid of gallium, iron, nickel, and silicon exposed to a gas of carbon-rich methane as well as hydrogen, scientists report April 24 in Nature. The process also needs lower temperatures than HPHT: 1025° C. The addition of silicon seems to start the initial stages of growth, allowing a small amount of diamond to form. Physical chemist Rodney Ruoff says this can help the rest of the crystal to grow.
The interest in diamonds isn't just for jewelry. Scientists can use diamonds for a variety of purposes including detecting magnetic fields to discovering new subatomic particles (SN: 9/19/22; SN: 6/17/19). This new process could make producing these materials easier. "The syntheses don't have to rely on expensive or complex equipment," says Ruoff of the Institute for Basic Science Center for Multidimensional Carbon Materials in Ulsan, South Korea.
Another way to create lab-grown diamonds, called chemical vapor deposition, or CVD, happens at low pressures, with a vapor of carbon-rich gas being deposited on a surface. Unlike CVD and HPHT, the new method doesn't use a small initial diamond to start the growth.
CVD and HPHT are widely used in the jewelry industry. It's not clear yet if the new technique will produce diamonds for jewelry.