Kate Nichols leans her delicate face against the glass of a chemical fume hood in a University of California, Berkeley lab, peering into a beaker filled with a pale yellow liquid—“like a well hydrated person’s pee,” she says, laughing. The yellow brew is a fresh batch of silver nanoparticles. Over the next week, the liquid will turn green, then turquoise, then blue as the particles morph in shape from spheroids to prisms under the influence of time and fluorescent light. Post-docs and grad students elsewhere in the nanotech lab are synthesizing nanoparticles for research on artificial photosynthesis and quantum dot digital displays. But not Nichols. She isn’t a scientist, but an artist, gripped by color.
About 15 miles away, in her studio in San Francisco’s Mission District, brightly colored pigments sit on a crowded shelf next to the nanopaints she made in the lab: vials of yellowish and brown solutions containing varying sizes and concentrations of silver nanoparticles. On a nearby wall opposite a large oil painting of close-up fish scales hangs a group of small sculptures she calls “Figments.” Each is made of two triangular pieces of glass covered in nanoparticle paint, and joined by a hinge. They look like eerie birds, or perhaps mutant butterflies, brightening or darkening depending on the light around them and the angle of the viewer, so that they seem to weave in and out of perceptual awareness.
The scene at her studio is in some sense not so different from the lamp-lit workspace of a Renaissance painter experimenting with new colors. “A lot of pigments were discovered by happy accidents,” explains Philip Ball, author of the book Bright Earth: Art and the Invention of Color. “We understand these mechanisms now, but then it was hands-on experimentation. It seems like [Nichols is doing] the same now with these [nanomaterials].” It’s doubtful any Renaissance painters, though, had an apprenticeship quite like this: Nichols, 34, has spent seven years as artist in residence at a world-class nanotech lab, mastering technically challenging synthesis techniques and learning about colloidal chemistry and optical physics. In the process, she has transformed not just her art, but also the way she looks at color itself.
Nichols’ journey from the studio to the lab has been driven by what she describes as an “almost maniacal obsession with mimesis.” As a young figurative painter, she was intrigued by the ability of Northern Renaissance painters to capture the luminosity of human skin. So in 2002 she left Kenyon College to study 15th-century oil painting and paint-making techniques as an apprentice to artist Will Wilson in San Francisco. She learned to make her own paints by mixing pigments with oils she concocted out of linseed oil, lead oxide, and mastic resin, following centuries-old recipes.
Around this time, Nichols became fixated on the Morpho butterfly. Entranced with its flickering blue-green iridescence—the hue shifting subtly as the insect flits through the air—she yearned to capture this luminous marvel of nature on her canvas. But how? She remembered what a math professor had explained to her in college: The Morpho’s color is structural. It doesn’t arise from pigmentation, but from the light scattering off nanoscale structures embedded in its wings.
Pigments absorb particular bandwidths of light depending on their chemical composition; whatever is not absorbed is reflected, and it is this reflected light that determines the color we perceive. Structural color isn’t chemical: Instead, tiny structures, often smaller than a single wavelength of light, redirect and slow light waves down, causing them to interfere with each other in ways that depend on the shape, size, and spacing of the scattering structures, as well as on the angle of the incoming light and the position of the observer. In the case of the Morpho, these structures scatter blue light most strongly; its hue shimmers and shifts to lighter or darker blue as the butterfly moves, producing iridescence. Structural color is also at work in peacock feathers, fish scales, and beetle casings.
“I think a scientist is on a personal journey that is very similar to what an artist takes. They each have to struggle to find what is truly beautiful.”
Nichols often had the radio on while painting in her studio, and in 2007 heard reports about developments in nanotechnology research on NPR. She began to wonder if it would be possible to make nanomaterials in the lab that would allow her to use structural color in her art. Using her oil technique she could already paint a photorealistic depiction of a Morpho butterfly, but she wanted a deeper mimesis: a blue created from the interaction of light with nanostructures akin to those on the butterfly’s wings.
On a whim, she wrote to Paul Alivisatos, a nanotechnology expert from UC Berkeley. To her surprise, after just one interview he invited her to join his lab as artist in residence. It was “a very easy” decision to take on Nichols, Alivisatos told me. “I think a scientist is on a personal journey that is very similar to what an artist takes. They each have to struggle to find what is truly beautiful. I always respect people who like to take those quests.”
Still, when Alivisatos first showed her the wet lab and realized she had never even been in a lab before, he “thought she might get cold feet.” The techniques involved in synthesizing nanomaterials “are quite demanding,” said Alivisatos, who is also the Director of Lawrence Berkeley National Lab. “But she’s been remarkably persistent.”
After joining the lab in late 2008 Nichols spent long days tagging along with “very generous” graduate students and post-docs. One of those was Jill Millstone, now an assistant professor of chemistry at the University of Pittsburgh. Nichols, who was 27 at the time, hadn’t studied any chemistry since high school. But Millstone said she “picked up on all of the nuances very quickly” regarding everything from mathematical calculations to pipette technique. “She embraced the language of science and the spirit of the way we approach problems,” Millstone added. “That made her melt into the fabric of the lab. She didn’t stick out as an artist.” For her part, Nichols says she found the learning process in the lab to be “similar to her experience of being a painter’s apprentice.”
At the core of her training were nanoparticles, microscopic particles generally measuring between one and 100 nanometers (one-billionth of a meter). To put this infinitesimal realm into perspective, a water molecule measures about 0.3 nanometers; a human hair is approximately 90,000 nanometers wide. Nanoparticles of metals (such as silver or gold) or semiconductors (such as cadmium sulfide or silicon) exhibit different, electrical, physical, and (especially interesting for Nichols) optical properties than they do in bulk. In addition, the way they interact with light can be controlled by changing their size and shape. To make such tiny structures in a controlled and repeatable way takes a combination of theory and technique, physics and chemistry, and meticulous attention to detail. Nichols understood that she had an opportunity to create materials that would serve as “a bridge between the nanoscopic and visible worlds”—but she was starting from zero.
It’s like a quest. You understand, then you don’t. It’s almost mystical.
She began to fill her lab notebooks with chemical equations and extensive notes for synthesizing nanoparticles made from a variety of materials, including silver, gold, and cadmium. Eventually she settled on silver as her primary material because of its safety and the colors it produced. At first she focused on prism-shaped silver nanoparticles, which displayed beautiful blues and greens in the vial. But she was “disappointed that it looked great in the bottle but if I put it on a glass surface it looked like my dirty windshield.” She couldn’t figure out how to get the silver nanoprisms she had made to adhere to glass the way she wanted. And after a year of solid lab work, she didn’t have any art to show for it. “I had to get knocked on my ass,” she said, remembering the many frustrations.
Finally, in late 2009, she decided to “listen to the particles.” Instead of using them like paint, she suspended them in sealed glass capillaries, which she attached to each other to create sculptures. They looked a little like ethereal organ pipes, or perhaps clusters of icicles, filled with turquoise liquid. Over time, as light caused the prisms to take on a more rounded shape, the particles turned blue.
The sculptures were a start, but she wasn’t satisfied. She continued to experiment and eventually found a way to create a paint based on another kind of silver nanoparticle, shaped like soccer balls. The key was their compatibility with a medium. Their specific surface chemistry meant she could suspend the pseudo spheres in organic solvents such as hexane, and apply them to glass. “The important step for her was getting to the stage where she could develop the material,” Alivisatos said. “First, she had to make the material and then have a process where she could embed it so it was stable. Just getting all that to work was hard.”
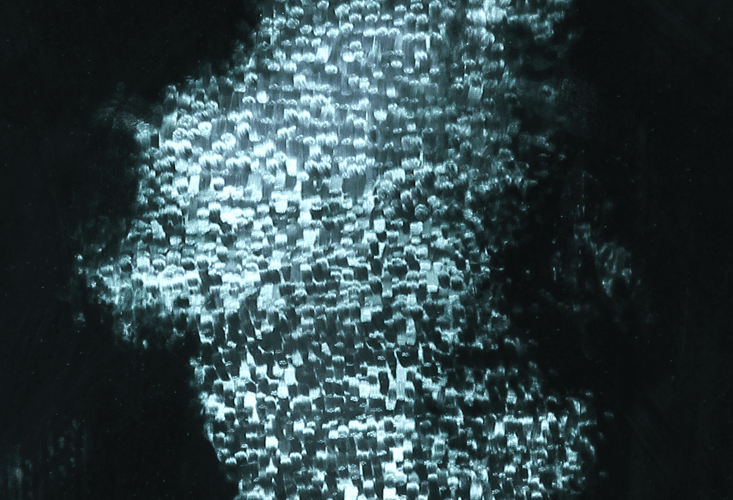
By late 2010 Nichols was able to consistently make silver nanopaints and start experimenting with them artistically. The Leonardo Museum in Salt Lake City learned about her experimentations through her 2010 TED talk and commissioned her to create a series of art works, which she called “Through the Looking Glass.” They consist of abstract, ghostly pools of brown, gold, orange, and purple colors on several superimposed pieces of glass, like large lab slides. These colors shift depending on where you stand and on the light falling on them, because the particles in the nanopaint transmit light in the brown-orange range, but scatter blues and purples. They tend to appear only blue and green on glass with a black background, which eliminates transmitted light, an effect she would explore later.
Success for a nanoscale scientist veers toward precision. The goal of synthesis is to create nanoparticles with the narrowest possible size distribution, allowing the most exact expression of a specific color for the highest-performing bio-imaging tool or information display or laser. Today artists can also get very precise. Ultramarine, one of the only materials available for producing blue pigment in the Middle Ages, was made from grinding up lapis lazuli, a precious stone that was only available from what is today Afghanistan. It was rare and extremely expensive. Now a fine artist has many blues to choose from for oil, acrylic, and watercolor. An industrialist in need of a blue for a Frisbee or toothpaste tube can dip into the over 9,000-page Color Index International. As Ball points out, poetic names such as Prussian Blue are in the minority, having been joined by chemically informative names such as C.I. VAT RED 13, a kind of red. “The ambiguities of older terminology are banished, and undoubtedly some of the magic goes with them.” The quantum dot industry also uses a prosaic naming system for the hundreds of colors in its quantum gamut: the letters QD, plus a number denoting its wavelength. QD680, for example, “is not a very beautiful name like ultramarine,” Alivisatos said. “But it does tell you exactly what it is.”
Nichols, on the other hand, brought her artist’s sensibility to the lab. The more she explored nanocolor, the more she was intrigued not by its precision, but by its ambivalence. The colors she coaxed out of the lab and into her artwork can’t be described with a single number. Her silver nanopaints are a purposefully “dirty” mix of particles of different sizes, varying from about five to 30 nanometers and scattering light over a range of the blue-green spectrum; she controls the distribution with a centrifuge. Some of her paints include gold as well, which adds reds. In a sense, each vial of Nichols’ paint contains a palette of colors, a mix of particles that interact with light in a variety of ways. Nichols likens the distinction between her “dirty” mixes and the formulas used for scientific purposes to the difference between sound reverberating through a wooden piano and a synthesizer. She’s attracted to the former, “the messier signal that comes with things that exist in the physical world.”
Nichols is, as far as I have been able to determine, the only artist to synthesize her own nanopaint in the lab. But what Nichols calls “nanocraftsmanship” has a long history. Nanoparticles of gold and silver have been used in art for thousands of years in ceramic glazes to lend iridescence and, most famously, in stained glass. The vibrant colors of the windows in medieval churches such as Sainte-Chapelle in Paris that glow in intense hues are created by nanoparticles embedded in the glass—silver particles for yellow and gold for ruby red. The color of the windows changes, as well, with the time of day and angle of light striking the particles.
The Lycurgus Cup, a glass chalice made by the Romans in the fourth century A.D. that today sits in the British Museum, looks green under reflected daylight, and red when light is transmitted through the cup. By 1990, scientists discovered that Roman craftsmen had embedded colloidal gold and silver nanoparticles into the glass, and that the effect was due to the same phenomenon (called surface plasmon resonance) that Nichols plays with on the Berkeley campus. These early nanocraftsmen arrived at their technologies through experimentation, and could not have known the science behind it.
Nichols also includes photography and Victorian mirror making, which involves a silver-based recipe “strikingly similar to my nanoparticle synthesis,” in the lineage of nanocraftsmanship. The silver particles in these processes aren’t as small as those in her nanopaints, but they have a similar ambivalent, multifaceted relationship with light.
She thinks about a material’s “particular relationship with light” instead of its color.
As artists begin to intentionally explore the artistic possibilities for using synthesized nanoparticles in their art, exciting new questions about color and how we perceive it will arise. “I think there’s no doubt that the number of different colors that can be purposefully made is much larger,” Alivisatos said. But what artists will do with this expanded palette is still an open question. “We’ve only begun to explore the ways these effects could be used artistically to communicate with color,” said Ball, “and to do everything that artists do to make us think about the visual world around us.”
Sculptor Anish Kapoor hasn’t ventured into the lab like Nichols but he has recently added a “super black” commercial nanopaint to his palette. Made by Surrey NanoSystems out of vertically aligned carbon nanotubes that trap over 99 percent of incoming light, “Vantablack” has applications such as camouflaging military aircraft. Kapoor has compared looking at the nanopaint to staring into a black hole. He told the BBC that a space painted with the substance is “so dark that as you walk in you lose all sense of where you are, what you are, and especially all sense of time.”
Nichols’ emphasis on the ambivalent qualities of metal silver nanoparticles could tickle the senses in other ways. Scott Taylor, who is a chief operating officer at Maxis, a division of Electronic Arts, and thinks about perception for his work on the SimCity videogame series, was so attracted to the dynamic nature of her art that he has bought several pieces, including one of the “Figments” series. He said he loves that how it looks depends on “whether I see it out of my peripheral vision or straight on, how the light hits it, what I am thinking at the moment.”
Nichols’ investigations into structural color have the power to inspire the same sense of wonder that her inspiration, the Morpho butterfly, often does. Her pieces at The Leonardo Museum were very layered, said Jann Haworth, the creative director. “First people saw the beauty, but then they went deeper, into the science of how something in nature could create such magic. It’s like a quest. You understand, then you don’t. It’s almost mystical.”
The nanopaints Nichols has developed so far are only a nano-sized sampling of the artistic possibilities in this new color realm. “If we’re seeing new visual effects who knows how we will respond to them,” Ball said. “We don’t know how that’s going to be perceived.”

Back in her studio, Nichols is working with some nanoparticle paint on a piece of glass. It’s hard to believe that the yellowish liquid she pours out was made in Alivisato’s nanotechnology lab. It looks like a thin stain or maybe a kind of finger paint—and she treats it as such, letting it flow onto the glass and then manipulating it with broken pieces of glass, and her gloved hand. When she feels the piece is done, she’ll cover it with another piece of glass. She has learned to sense how the pieces will look after the solvent dries and under different light conditions. But she’s often surprised, “and that is what’s delightful and maddening about working with this material,” she laughs.
I ask her about some dark glass pieces on the wall. Unlike the clear pieces she showed at The Leonardo, these have a black backing to block transmitted light. They look a little like ghostly black-and-white negatives with flickers of blues and greens in different streaks and patches, depending on how you look at it. As I always do when I observe her nano art, I walk around, exploring how it changes. Then I lean in close. “What color is this anyway?”
She points out a “blue like a gaslight, something more like the silver of silverware, and a green quality but still in blues.” But, she adds, “the question is insufficient.” After working with nanoparticles that look “yellow here and blue there, that change right in front of my eyes,” she thinks about a material’s “particular relationship with light” instead of its color. There is no simple answer to my question. In the last several years, she has brought this sensibility into all of her artwork. Whether painting in traditional oils, making mirrored surfaces, or working with her nanopaint, Nichols crafts “surfaces to catch, scatter, bend, and transmit light in ways that allow us to experience light’s ambivalent nature,” as she described in her 2012 TEDx talk.
As I continue to circle around the dark glass piece in the afternoon light of the studio, I see new forms and colors that I hadn’t noticed before. Like a Morpho butterfly, the piece is alive with light. It makes me think of all the marvelous things that happen when the sun’s rays fall to Earth.
Jeanne Carstensen is a writer in San Francisco. Her work has appeared in The New York Times, Salon, Modern Farmer, and other publications.