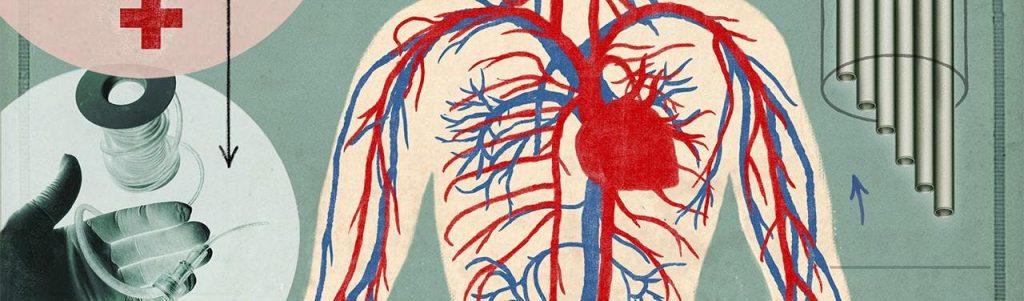
Even in the 1990s, the procedure seemed primitive. Laura Niklason watched it repeatedly as a medical resident at Boston’s Massachusetts General Hospital. When patients undergoing cardiac bypass surgery needed a new vessel to bypass the blocked one, surgeons would often steal a vein or artery from elsewhere in their body: a leg, usually, but sometimes an arm. If those options failed, maybe doctors would extract one from the patient’s abdomen.
Niklason was shocked that there was no viable alternative. Beyond the pain, the patient now had two regions to heal—and twice the potential for infection. The surgeons were harming one part of a patient’s body to save another. There had to be an alternative, she thought. What if she could grow replacement human blood vessels on demand?
It sounded crazy at the time because nothing like that existed. But the vessel-harvesting procedure haunted her. The surgery continues to this day for a number of diseases: when cardiac patients have blocked veins and arteries, or when dialysis patients need new vessels after theirs have been repeatedly punctured as surgeons access and cleanse their blood. Synthetic arteries made of a Teflon-like material are on the market, but these are prone to infection and inflammation when the immune system attacks the non-human tubes. As a result, patients frequently undergo repeat operations to replace the implants.
A lab-grown organ, built by human cells and made up of the body’s natural construction materials, could assist in more than 500,000 blood vessel replacement surgeries every year. “The need for replacement arteries is really pretty staggering,” Niklason says. The market for such arteries, she adds, reaches hundreds of millions of dollars a year.
Lucky for those of us who have yet to undergo these kinds of operations, Niklason persevered despite technological hurdles and push-back from the scientific community. Finally, her living tubes are being implanted in humans.
Back in the ’90s, Niklason confided her interest in lab-grown veins to her mentor in Boston, Charles Vacanti. Vacanti had an interest in growing organs from scratch, and he would soon achieve particular fame for the “earmouse,” a human-shaped cartilage ear implanted under a mouse’s skin. Vacanti referred Niklason to Massachusetts Institute of Technology (MIT) tissue engineer Robert Langer. Niklason, who had completed a medical degree and a Ph.D. in biophysics, joined Langer as a post-doc.
In Langer’s lab, Niklason began work on something that, at the time, few people in the world had considered. She knew that cells in our blood vessels were subject to the push and pull of flowing blood every second of every day. Without feedback from flowing liquid, blood vessels lose their strength and stability and eventually waste away. Therefore, to convince human cells to build a stable, strong, flexible tube, one that could be surgically implanted and would thrive in the body’s tumultuous environment, she would need to design an apparatus to pulse the growing cells like the human body does.
It seemed logical to her. But in the mid-1990s, this was anything but logical. Most biologists who were trying to coax cells into forming tissue attempted to do so through entirely chemical means. Figure out just the right cocktail, the magic blend of ingredients, and feed that to cells to get them to behave the way you wanted.
Chemical nutrients did trigger cells to multiply, but that wasn’t enough. Cells also need to lay down the external matrix that supports and houses cells and forms a three-dimensional structure. To build this scaffold, Niklason considered cues that come from their surroundings. “We’ve evolved in an environment filled with physical forces,” says Niklason. “Billions of years of selective pressure have forced our cells and our organs to thrive within a complex mechanical milieu that’s distinct for every tissue.” By copying this, she says, “we’re harnessing all those years of evolutionary selection and producing tissues that can function just as native arteries do.”
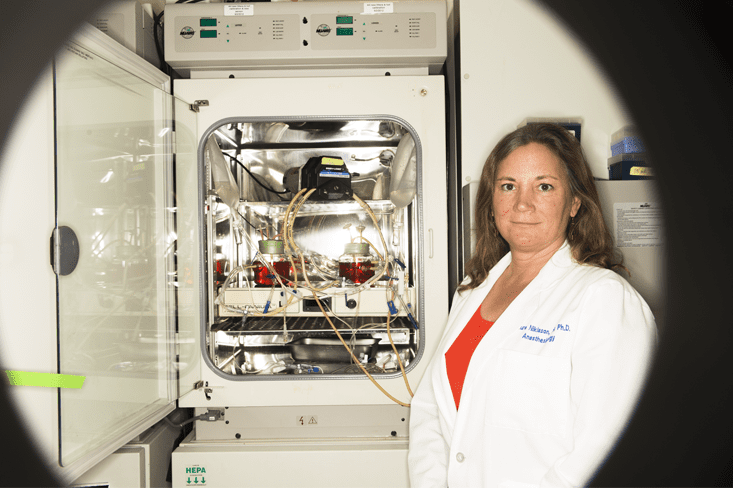
Had Niklason not worked in close proximity to a few other iconoclastic bioengineers, her notions about pulsating internal environments might have been lost to history. She was a post-doctoral researcher, after all, with all the pressures of an academic career ahead of her. Yet in Boston, she was not alone.
Another Boston-based bioengineer, Don Ingber, had been marginalized as well. A sculpture class he took as an undergraduate had inspired an odd hypothesis. He predicted that the forces within a body are channeled all the way to the genome in a cell’s nucleus, where they influence how genes are expressed. Ingber named his theory “tensegrity,” after an architectural term coined in the 1960s.
Tensegrity wasn’t understood or accepted when Ingber proposed it, he says. “I’d present these ideas when I was a grad student, and [scientists] would say, ‘Everything is chemistry. Mechanics went out with vitalism [the idea that there’s a vital inner force that creates life] at the end of the last century. This is ridiculous, your work is bullshit.’ ”
While Ingber proclaimed the importance of mechanical stresses on cells, Robert Langer was well on his way to creating one of the country’s preeminent tissue engineering labs at MIT. Boston was awash with ideas about how to regrow parts of the body. Niklason, a young researcher, was lucky to be there, in an environment where her unconventional ideas weren’t tossed aside. “The field of regenerative medicine was much more nascent at the time,” she recalls. “It’s fair to say that it was kind of lunatic fringe, not a lot of people took it seriously.”
Langer did. He says Niklason’s approach made perfect sense to him. Other researchers in his lab were studying the influence of stresses and feedback on cells, but no one was forging Niklason’s path, that of trying to copy the innate pressures of the body. Yet even with his support, Niklason says, “The biggest challenge was that there was really no road map. It’s not like you could go to a textbook and read the chapter on how to grow a new artery.”
“Billions of years of selective pressure have forced our cells and our organs to thrive within a complex mechanical milieu that’s distinct for every tissue.”
Instead, she turned to the textbook of the body. “I knew from my medical school studies that the action of the heart was going to be very important to mimic in the laboratory.” She smiles wryly. “Getting that right was actually hard.”
Hard, but not impossible. After a couple of years, she was able to fashion a functioning machine, which she calls a bio-incubator, to grow a vessel. She implanted cells from cow blood vessels into an airy, felt-like, biodegradable scaffold made of a common surgical material called polyglycolic acid. Then she immersed the system in a cocktail of growth factors and other chemicals that fed the cells as they reproduced. The scaffold surrounded a silicone tube, and liquid pulsed through the tube, pushing and contracting, tricking the cells into experiencing the beat of a heart.
What happened next is what biologists had failed to achieve in the past. The cells responded by excreting the building-blocks of a vein: collagen and elastin, along with minute amounts of other proteins, in an intricate interlocking pattern that provided strength and flexibility even beyond that of a natural vessel. As the creation grew, the felt-like mesh disintegrated. The silicone inner tube was removed. The result was a lab-grown blood vessel, created by animal cells. Niklason and Langer published the results in Science in 1999, in a paper called “Functional Arteries Grown In-Vitro.” Since then, dozens of labs around the world have followed Niklason’s lead.
But Niklason’s ultimate goal was to implant the vessels in people, which is far riskier than toying with the tubes in a lab. If one burst under pressure, it could potentially kill a person.
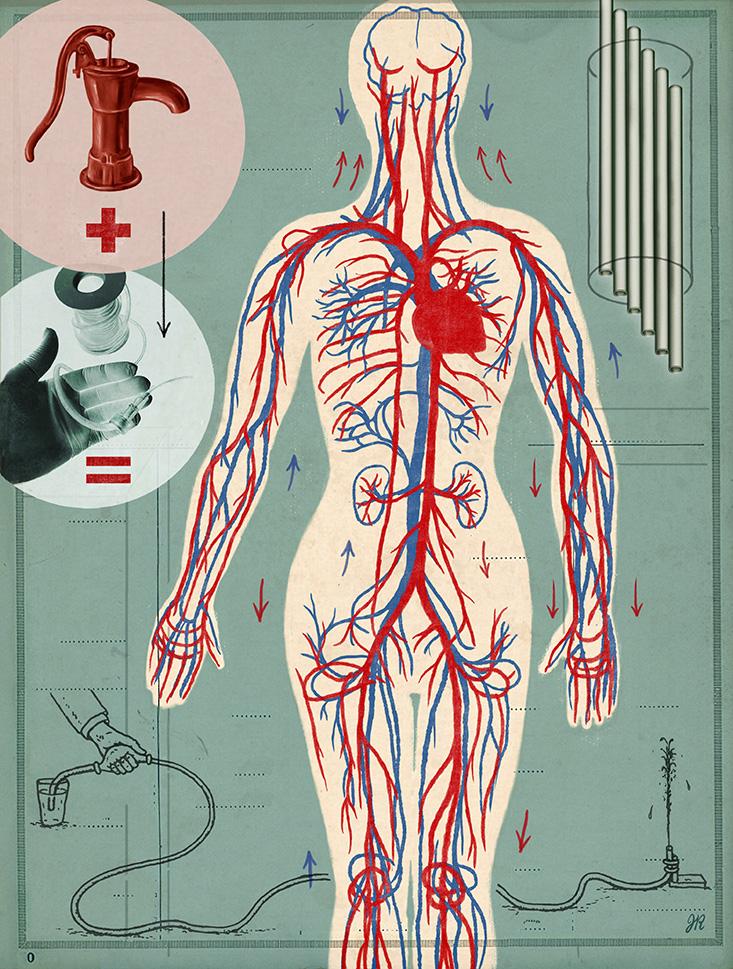
So began the next stage of Niklason’s journey towards implanting living vessels. She moved to North Carolina to continue her research as an assistant professor at Duke University. Six years later, in 2004, she and her first Ph.D. student, Shannon Dahl, along with Juliana Blum, who had been a post-doc in her lab, founded a company called Humacyte to commercialize the lab-grown veins.
Niklason’s original idea was to grow a patient’s new vessel with their own harvested cells. But this proved difficult because patients often had weakened blood vessel cells, which failed to grow hearty new veins. Instead, the team decided to use healthier cells from the veins of organ donors. To avoid those cells being rejected, she decided to rinse away the donor cells after they constructed a protein scaffold. “The whole concept of taking all the trouble to grow an artery and then washing the cells away seemed very counter-productive and counter-intuitive,” Niklason says. But she and her team did it regardless.
Next, she toyed with the bio-incubator to be sure that its pulses mimicked the pressures the vessel would face in the human body. Her research became more complicated when pigs and dogs rejected the human-made implant. But in primates, the final test before people, the vessels worked.
After years of research, the team felt sure they had devised a method to create human cell-grown blood vessels. Niklason calls them “stealth tissues,” designed to fool the patient’s body into recognizing the new tissue as its own. Evidence from the baboons even suggests that the patient’s own cells colonize the foreign tissue after it’s implanted.
This year, Niklason’s hollow, milky-white blood vessels are being implanted in patients enrolled in clinical trials in Poland and the United States. The first stage of testing will determine the vessels’ safety. The next stage of trials will demonstrate whether the tubes represent an improvement over existing techniques.
A handful of other bioengineers have converged on techniques in the same vein. Simon Hoerstrup, now head of the Swiss Center for Regenerative Medicine at the University of Zurich, has independently developed tiny vessels for babies. Hoerstrup had come to research after years as a pediatric cardiologist, where he had seen babies born with heart problems that were difficult, if not impossible, for surgeons to fix. His reactions were not unlike Niklason’s responses to the surgeries she saw in the 1990s.
“The field of regenerative medicine was much more nascent at the time,” she recalls. “It’s fair to say that it was kind of lunatic fringe, not a lot of people took it seriously.”
Over the same interval in time, Hoerstrup also created a system that mimics a heartbeat. The baby’s cells are first harvested and then grown on a scaffold that sits in a bath of chemicals, and that beats with the rapid tap of a baby’s pulse. Within three weeks, the researchers create a tiny working vessel, rich with living cells that can be used to fix the baby’s heart and will grow with the infant as their heart deposits additional cells and tissue. Hoerstrup’s technology will enter clinical trials later this year.
In the meantime, Paolo Macchiarini, at the Children’s Hospital of Illinois, has created artificial tracheas in a process that replicates airways, outside of the body. And Ingber—once the odd-man-out with his talk of tensegrity—founded the Wyss Institute for Biologically Inspired Engineering at Harvard University in 2009. The term “tensegrity” faded, but the phenomenon it described is now treated as common knowledge. The Institute has been lauded for organs-on-a-chip, a piece of technology that combines cells and microfluidics to mimic parts of our body for drug and toxicity testing. In the “lung-on-a-chip,” for example, air whooshes through tiny channels to replicate exhales and inhales, prompting the lung cells embedded in it to act as they would in the body.
These are promising first steps, though scientists are still years, perhaps decades, away from building entire complex organs such as a heart or a liver from scratch. Simple structures, like cells used for drug development and tubes to replace vessels, are a start.
A completely different technique for artificial organs has recently captivated the public: 3D printing. Scientists recently printed liver cells, but when they eventually attempt to build vessels and organs, the next step will again be to add the type of stressors that Niklason, now a biomedical engineer at Yale University, developed over the past 20 years.
In 2017, care for cardiac and dialysis patients might transform into something less gruesome than the current approach. If Niklason’s vessels prove themselves in clinical trials, they’ll enter the market by then. All our cells and all our organs evolved in complex, force-filled environments, says Niklason. Finally, after 20 years, she’s figured out a way to copy it.
Cynthia Graber is an award-winning print and radio reporter based in Somerville, Mass.